41 bh2 molecular orbital diagram
Free essays, homework help, flashcards, research papers, book reports, term papers, history, science, politics pause-rounded-fill. 00:00. 1x 1.25x 1.5x 1.75x 2x. Problem Details. Draw a molecular orbital diagram for Ar 2+. This ion has been observed in the gas phase. Calculate bond order and describe how the bond distance in this ion would differ from that in Cl 2. Frequently Asked Questions.
set '(Slater orbitals) by over 100 kcal/mole than either of two conformers of the 0012 topology. These 0012 structures have two BH2 groups joined by a single B-B bond, and therefore each boron has a vacant orbital (iv relative to the H2B-plane). We find (13) that the staggered conformer (D2d) is more stable by 13 kcal/
Bh2 molecular orbital diagram
Theoretical chemistry research group focusing on development of methods, and calculations in the areas of ionic liquids, photochemistry and catalysis Molecular Orbitals for Larger Molecules 1. Determine point group of molecule (if linear, use D2h and C2v instead of D∞h or C∞v) 2. Assign x, y, z coordinates (z axis is principal axis; if non-linear, y axes of outer atoms point to central atom)3. Find the characters of the reducible representationfor the combination of The π molecular orbitals in the order of an increasing energy are calculated. The molecular structure: π electron distribution, final charge distribution, final fractional bond ordor, bond length of each C-C bonds, are also calculated for the ground state. It is concluded that both of the tropylium ions have C 2e symmetry.
Bh2 molecular orbital diagram. (2) Determine the most stable molecular structure for CH2. Would you expect BH2 to adopt a similar structure? Hint: Use a correlation diagram (Walsh) to determine how the orbital energies change as a function of bending. (3) Construct the molecular orbital diagrams for (a) AB4 (Td case) and (b) AB6 (Oh case) where A is a first-row transition November 11, 2016 - As discussed in class the MO diagram for B2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond. Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: What molecule is illustrated by the following molecular orbital diagram? Atomic orbitals Molecular orbitals Atomic orbitals 2p 2p 2s 2s 2s Provide your answer below. but set the isovalue to -0.05. The resulting displayed volumes correspond to the molecular orbital 39 with an absolute value greater than 0.05, shown in figure 4. Figure 4: The highest occupied molecular orbital of methionine plotted at isovalues ±0.05 (left) and ±0.01 (right). 5. Following Molecular Orbitals Through a Trajectory
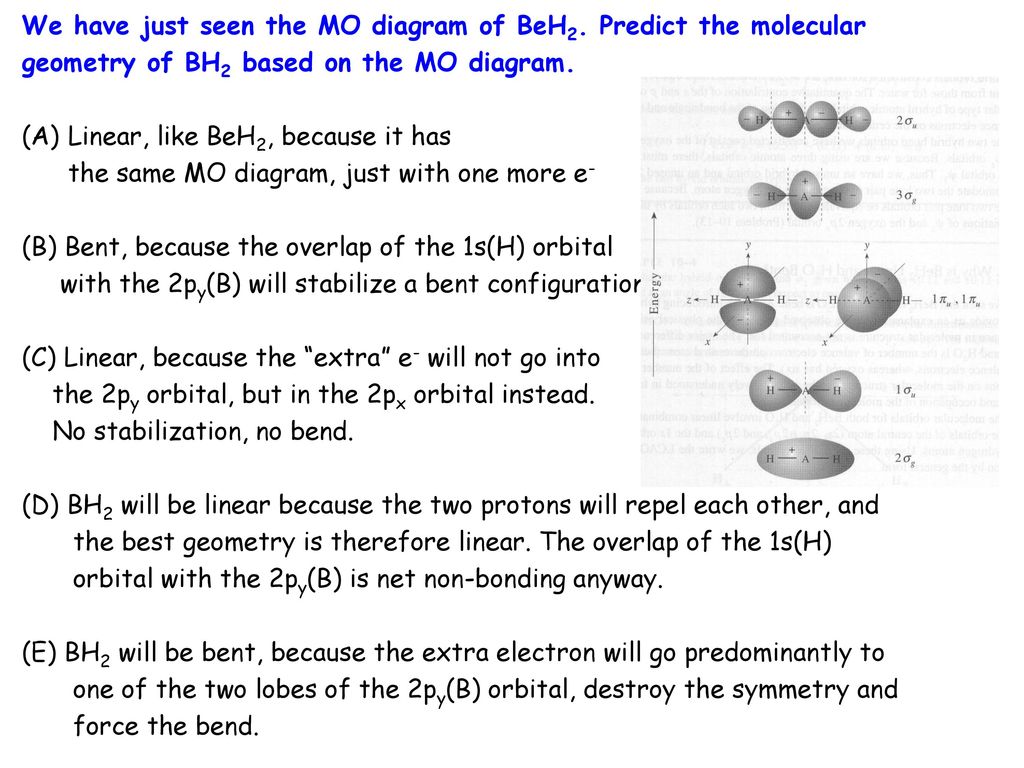
(i) Be2 molecule: The electronic configuration of Be(Z = 4) is: 4 Be 1s 2 2s 1 Be 2 molecule is formed by the overlap of atomic orbitals of both beryllium atoms. Number of valence electrons in Be atom = 2 Thus in the formation of Be 2 molecule, two outer electrons of each Be atom i.e. 4 in all, have to be accommodated in various molecular orbitals in the increasing order of their energies. BH2. Fig. The interaction of AOs can be represented on a MO diagram where the respective . BH2. 5. Bent. CH2. 6. Bent. NH2. 7. Bent. OH2. 8. Bent. For three or four. Walsh correlation diagram is a plot of molecular orbital energy as a function of some systematic change in molecular geometry. We have just seen the MO diagram of BeH2. Predict the molecular geometry of BH2 based on the MO diagram (Fig in McQuarrie & Simon). (A) BH2 will be linear, ... Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
(2) Determine the most stable molecular structure for CH2. Would you expect BH2 to adopt a similar structure? Hint: Use a correlation diagram (Walsh) to determine how the orbital energies change as a function of bending. (3) Construct the molecular orbital diagrams for (a) AB4 (Td case) and (b) AB6 (Oh case) where A is a first-row transition An advanced molecular orbital diagram of BeH2 (beryllium hydride) for the inorganic or physical chemistry student. August 23, 2017 - I'm trying to build a molecular orbital diagram for BF3 and I'm running into problems with irreducible representations on the F side. 2s for B has an irreducible representation of A1. 2p for B ha... Wiring Diagram Pictures - schematron.org
Question 1 (50 points total): Using coordinate system shown, derive a molecular orbital diagram (o + ) for the bonding between the carbon and nitrogen atoms in the guanidnium cation ([C(NH2)3]+). Assume that the peripheral nitrogen atoms use only symmetrically similar p-orbitals to interact with the central carbon atom (which can use all of its ...
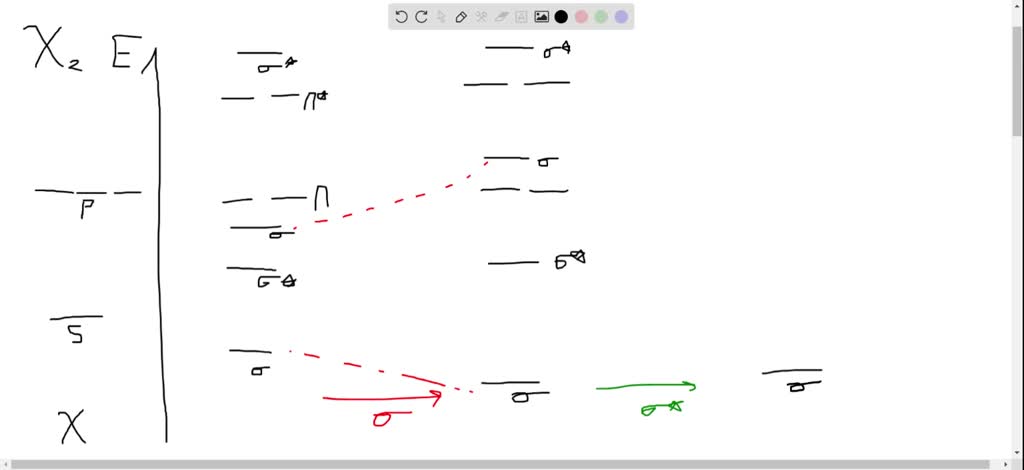
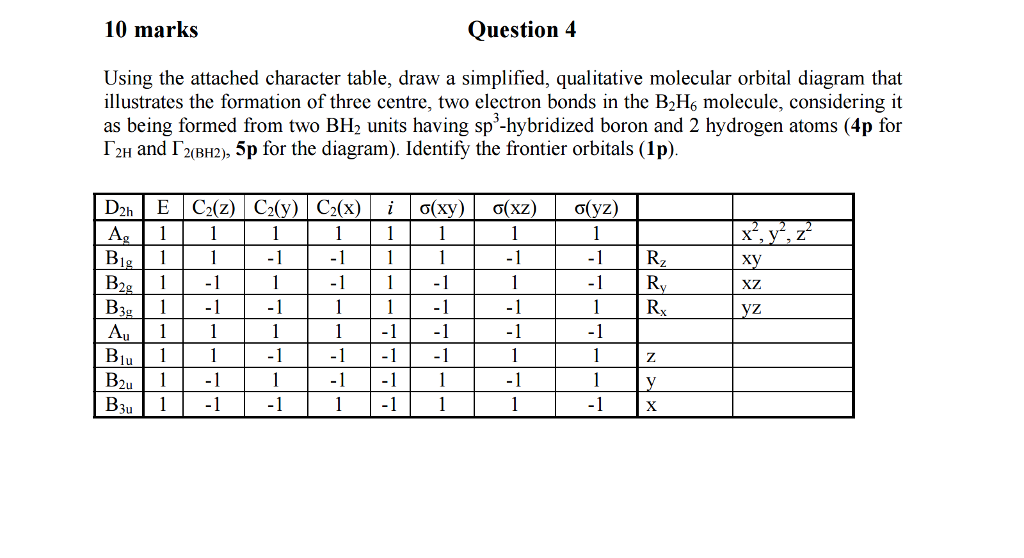
(15 pts) Consider the molecule B2H6. Generate a molecular orbital diagram but this time using a different approach that draws on your knowledge and ability to put concepts together. First generate an MO diagram for BH2. Sketch the highest occupied and lowest unoccupied MOs of the BH, fragment. These are called frontier orbitals.
What is the molecular geometry of BH2-? VSEPR theory would predict a molecular structure that was bent with a bond angle < 120 degrees AX2E. What is the Lewis structure of BH2? H-b(..)-b.
Solved Constructing A Mo Diagram For Bh2cl Construct A Stage 1 Mo Diagram Using Bh2 And Cl As The Fragments Assume That The Cl 3sao Is Non Bonding Show How The Mixed Orbitals Are
Answer to: What is the Lewis structure of NI3? By signing up, you'll get thousands of step-by-step solutions to your homework questions. 5 92. This is not the case. 3 Molecular orbital diagram of Ferrocene 9. BH2 angle. You can Molecular shape with 2 electron groups.
Answer to A. The Lewis diagram for BH2 is: H-B-H The electron- pair geometry around the B atom in BH2 is There arelone pair(s) aro...
Draw the molecular orbital diagram for:(i) Be2(ii) B2 and . Save Image. 29 Molecular Orbital Diagram Of B2 Wire Diagram Source
BeH2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. BeH2 is known as beryllium hydride or beryllium dihydride. It is an inorganic compound and comes under the category of alkaline earth hydride. It appears as an amorphous white solid at standard temperature and pressure. It also exists in polymeric form as (BeH2) n.
Download scientific diagram | Molecular orbital energy diagram for BH 3 from publication: A brief introduction to molecular orbital theory of simple polyatomic molecules for undergraduate chemistry students | Recebido em 18/7/11; aceito em 14/2/12; publicado na web em 15/6/12 A simple, four-step ...
Molecules Free Full Text Toward Exploring Novel Organic Materials Mp4 Dft Properties Of 4 Amino 3 Iminoindene Html
Beryllium hydride | BeH2 | CID 139073 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities ...
sp 2 Hybridization. The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp 2 hybrid orbitals and one unhybridized p orbital. This arrangement results from sp 2 hybridization, the mixing of one s orbital and two p orbitals to produce three identical hybrid orbitals oriented in a trigonal planar geometry ().
Things that can be determined from the Hückel molecular orbitals:! 4) Determine charge on a given carbon! Remember that the square of the coefficient value is related ! to the probability of finding electron density! q i = (electrons in orbital 1)(C i1)2 + (electrons in orbital 2)(C i2)2 + (for each filled orbital) ! Consider the allyl anion!
Polyatomic Molecular Orbital Theory Transformational properties of atomic orbitals Atomic orbital Transforms as s x2+y 2+z 2 px x py y pz z dz2 z2, 2z 2-x2-y2 dx2-y2 x2-y2 dxy xy dxz xz dyz yz S py • When bonds are formed, atomic orbitals combine according to their symmetry. • Symmetry properties and degeneracy of orbitals and bonds can be ...
Molecular Orbital Energies The orbital energies are given in eV, where 1 eV=96.49 kJ/mol. Orbitals with very low energy are core 1s orbitals. More antibonding orbitals than you might expect are sometimes listed, because d orbitals are always included for heavy atoms and p orbitals are included for H atoms.
a complex MO diagram: B2H6 ... MO diagrams combine two fragments. Symmetry fragments ... fragment =5e therefor keep up to b2 orbital z x y b1 a1 a1 b2. BH2.
February 8, 2016 - If you draw out BH2F, you'll see that is a trigonal planar molecule with B in the middle, and the 2 H atoms and the 1 F atom around it. In this case, the F atom is different from the 2 H atoms and can occupy 3 different positions around the central B atom, making the degeneracy (W)= 3.
3) Combine SALCS with orbitals on A For more complex molecules, there may be more than 2 symmetry equivalent types of atoms. In this case, MO diagrams can be constructed from the orbitals of two chemically reasonable fragments. For example, the bonding in [Cl 3Pt(C 2H4)]-can reasonably be considered in terms of the interaction between [PtCl 3 ...
for molecules'° the molecular orbitals are expressed as linear combinations of atomic orbitals, all molecular integrals including those over is orbitals are evaluated accurately, and the total energy of the system is minimized ... between BH and BH2 groups are more strongly bonded towards the BH group, and, secondly, the electron density is ...
In molecules with five or six valence electrons, e.g. BH2 or CH2, Walsh's diagramm suggests that orbital 3a1 harbours the additional electrons. This orbital would favour an bent conformation. In fact, all molecules with five or six valence electrons are angular.
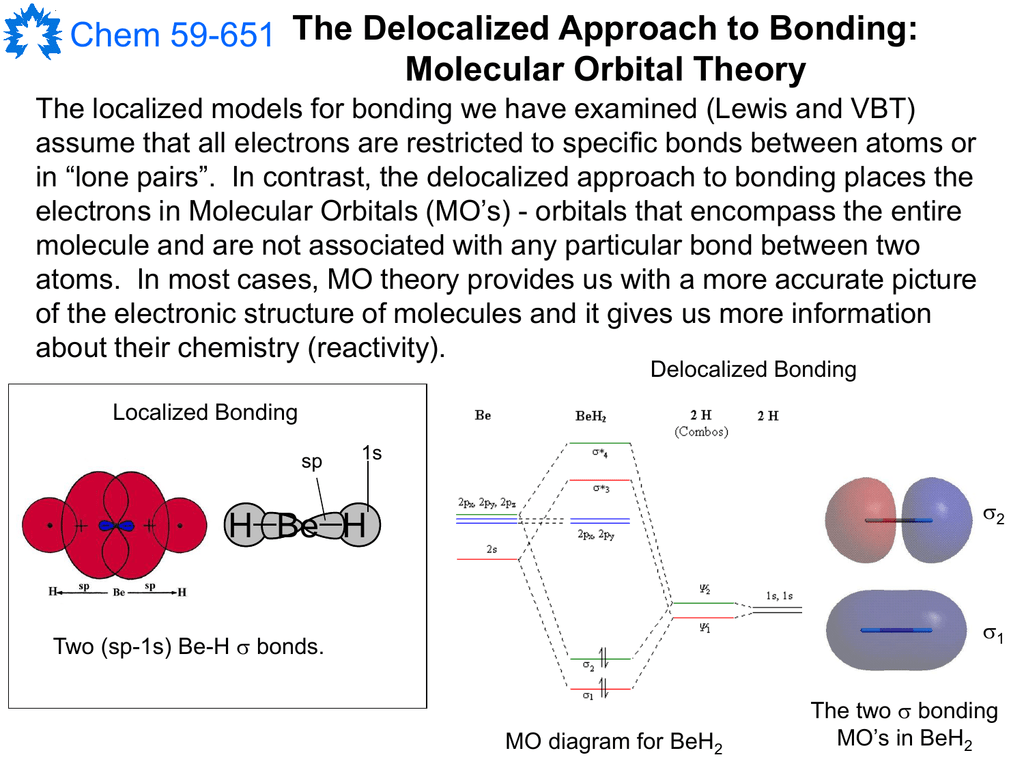
Here are the molecular orbitals for BeH2 from symmetry adjusted linear combinations.
(2) Determine the most stable molecular structure for CH2. Would you expect BH2 to adopt a similar structure? Hint: Use a correlation diagram (Walsh) to determine how the orbital energies change as a function of bending. (3) Construct the molecular orbital diagrams for (a) AB4 (Td case) and (b) AB6 (Oh case) where A is a first-row transition
Download scientific diagram | Frontier molecular orbitals (FMOs) of the tropylium cation (1); phosphatropylium cation (2) and 1,2-diphosphatropylium cation (3). from publication: molecules Effect ...
July 14, 2020 - Pople, John A.. Approximate Molecular Orbital Theory (Advanced Chemistry). New York: Mcgraw-Hill (Tx), 1970. Print., Phy Sci Engr Library QD461. P66
November 27, 2017 - Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons.
Plot MO energies and draw orbitals ... Walsh's correlation diagram: Plot course of MO energies with change in geometry ... e.g. BH2, CH2, NH2, H2O, H2S ...32 pages
Atomic orbitals are labelled 1s, 2s, 2p, 3s, 3p, 3d, etc. where the letter (s, p, d or f)indicates the orbital shape: Molecular orbitals are labelled 1σ, 2σ, 1π, 2π, etc. where the Greek letter (σor π) indicates the orbital symmetry: Molecular orbitals are generated by combining atomic orbitals:
I quickly take you through how to draw the Lewis Structure of BH4-, (tetrahydroborate ion). I also go over hybridization, shape and bond angle.
Draw Molecular Orbital Diagrams for the Be2 and the O2+ molecules and put the electrons in the resulting molecular orbitals. (6 pts) What are the Bond Orders for the Be2 and the O2+ molecules? 2 pts) Be2 B.O. = σ2σ*2 B.O. = 0 ... The BH2 fragment has two ordinary bonds made from two of the four sp3 hybrids and the H 1s orbitals. These BH2 ...
NH, NH2, BH, BH2 and BH3 by P. C. H. JORDANT and H. C. LONGUET-HIGGINS Department of Theoretical Chemistry, University of Cambridge (Received 4 August 1961) This paper describes a semi-emprical quantitative theory of the tow-lying electronic states of the radicals CH, CFI2, CHa, NH, NH,, BFI, BH2 and BHa.
Molecular Orbitals for Diborane, B 2 H 6. Jmol models of calculated wavefunctions. To view a model, click in a molecular orbital circle in the energy level correlation diagram shown Mouse Control of Models. Left mouse drag to rotate; Shift Left drag up or down to resize; Shift Right drag or ...
The wave functions, level energies and Mülliken population analysis of localized molecular orbitals (LMO's) for B4Cl4, 1,5-C2B3H5 and the closo-BnH2-n (n = 6-10, 12) are calculated by using the ...
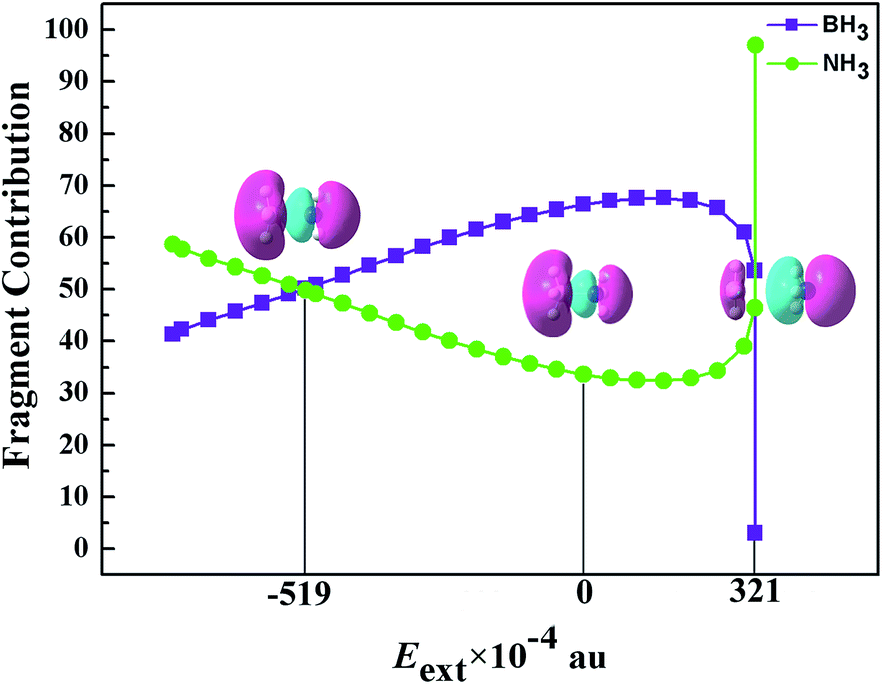
Ammonia Borane In An External Electric Field Structure Charge Transfer And Chemical Bonding Rsc Advances Rsc Publishing Doi 10 1039 C5ra10156e
The new orbitals thus formed are known as hybrid orbitals. More significantly, hybrid orbitals are quite useful in explaining atomic bonding properties and molecular geometry. Let us quickly look at the example of a carbon atom. This atom forms 4 single bonds wherein the valence-shell s orbital mixes with 3 valence-shell p orbitals.

Draw The Molecular Orbital Diagram For I Be2 Ii B2 And Predict Bond Order And Magnetic Properties From Chemistry Chemical Bonding And Molecular Structure Class 11 Assam Board
Would you expect BH2 to adopt a similar structure? Hint: Use acorrelation diagram (Walsh) to determine how the orbital energies change as a function of bending.(3) Construct the molecular orbital diagrams for (a) AB4 (Td case) and (b) AB6 (Oh case) where A is a first-row transitionmetal atom and B is a hydrogen atom. Hint: Use the same methodology employed in (2), but recall that the B atomsare not necessarily planar in this case.(4) Using a fragment orbital approach construct a molecular ...
Molecular species No. of valence electrons Shape Known shape of some AH 2 molecules FH 2+ 118 SH 2 92.1 OH 2 104.5 NH 2 103 SiH 2 93 CH 2 110 BH 2 131 Molecular angle species. D∞h C2v. Walsh diagram better overlap of H 1s orbitals poorer overlap of H1s with 2p x remains non-bonding First order effects. Walsh diagram mixing can occur between 2 ...
The π molecular orbitals in the order of an increasing energy are calculated. The molecular structure: π electron distribution, final charge distribution, final fractional bond ordor, bond length of each C-C bonds, are also calculated for the ground state. It is concluded that both of the tropylium ions have C 2e symmetry.
Molecular Orbitals for Larger Molecules 1. Determine point group of molecule (if linear, use D2h and C2v instead of D∞h or C∞v) 2. Assign x, y, z coordinates (z axis is principal axis; if non-linear, y axes of outer atoms point to central atom)3. Find the characters of the reducible representationfor the combination of
Theoretical chemistry research group focusing on development of methods, and calculations in the areas of ionic liquids, photochemistry and catalysis












0 Response to "41 bh2 molecular orbital diagram"
Post a Comment