41 fractional distillation phase diagram
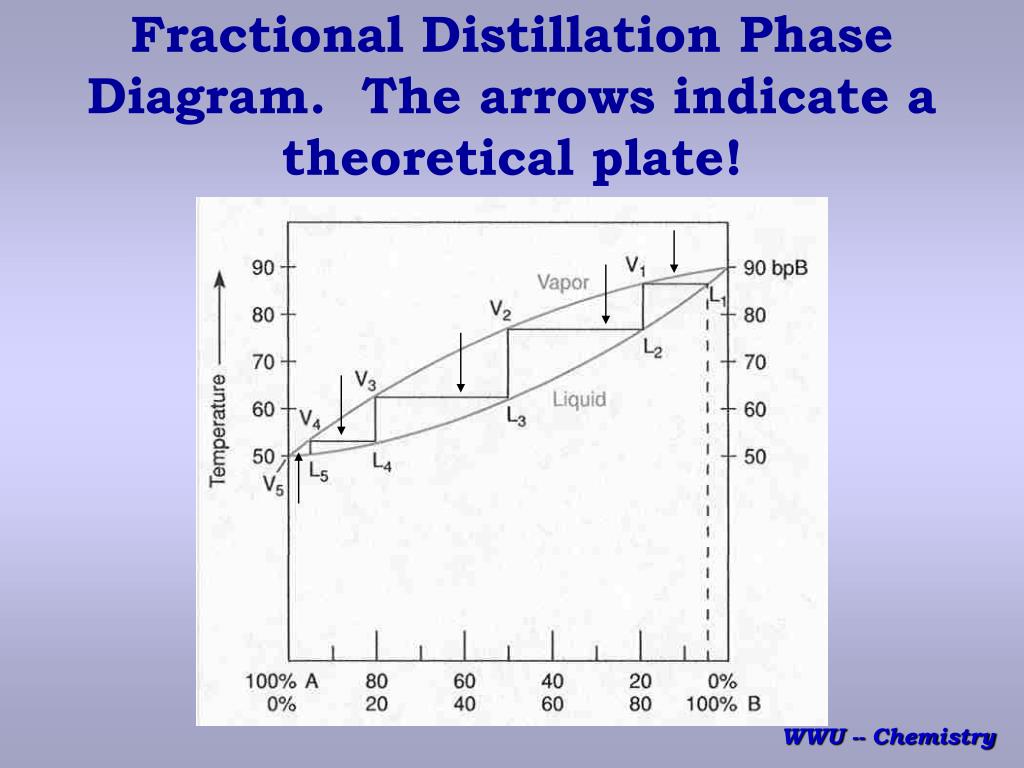
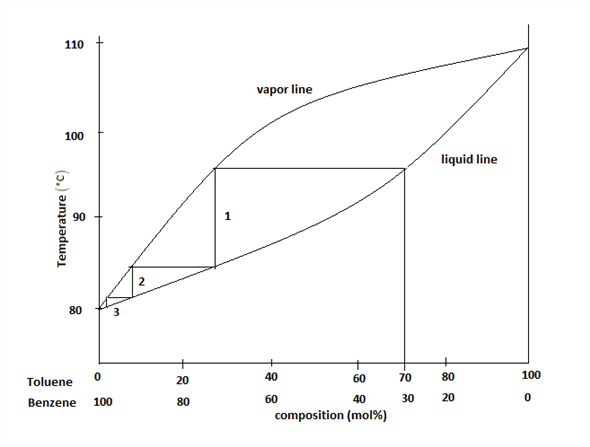
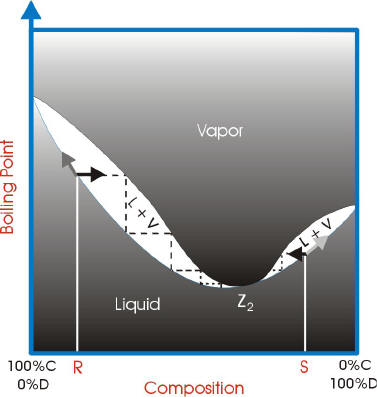
Feed condition Value of q Subcooled liquid >1 • q: ratio of the increase in molar reflux rate across the feed > 1 Bubble-point liquid 1 Partially vaporized L F /F reflux rate across the feed stage to the molar feed rate L L V V Dew-point vapor 0 Superheated vapor < 0 F q F V V q 1 CHM220 Distillation Lab Page 4 of 7 Figure 5b Fractional Distillation Phase Diagram. The arrows indicate a theoretical plate. Each vaporization is represented by a horizontal line connecting the liquid composition curve to the vapor composition curve. Each condensation is represented by a vertical line connecting the
Phase diagrams for fractional distillation. I learned about phase diagrams involving partial Vapour composition, temperature and composition of binary solutions from this website. You can find it if you scroll down to a little above the end. First it considers a binary solution of volatile liquids. The diagram involves boiling point on Y-axis ...
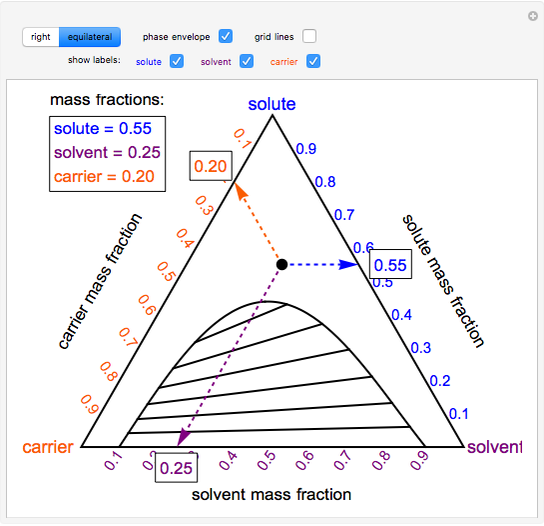
Fractional distillation phase diagram
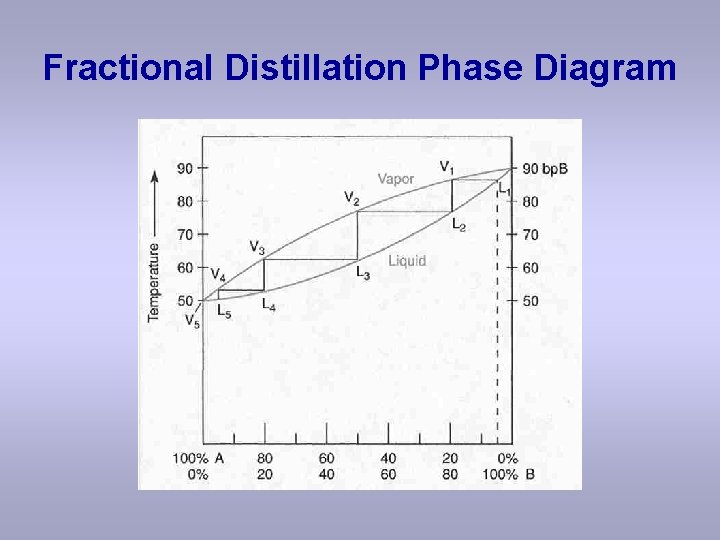
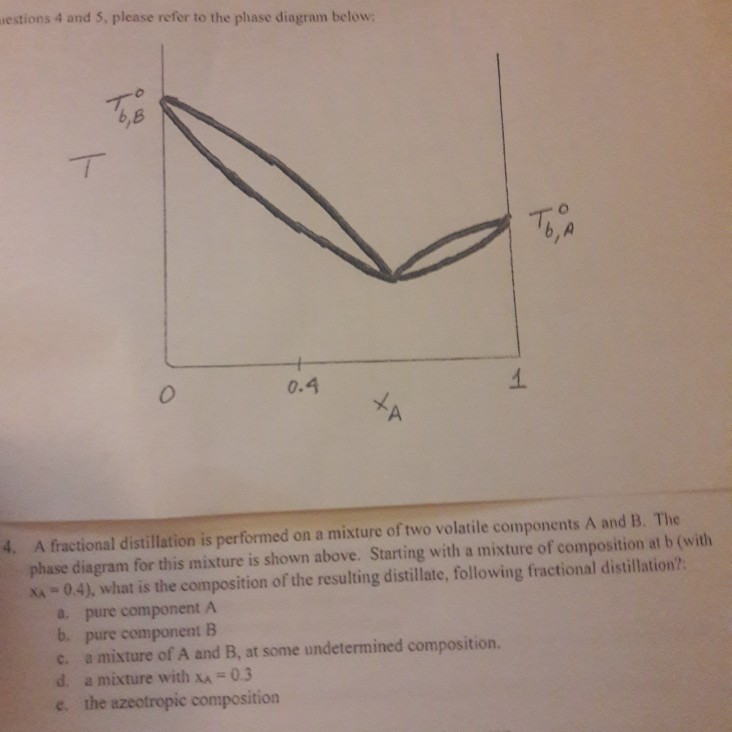
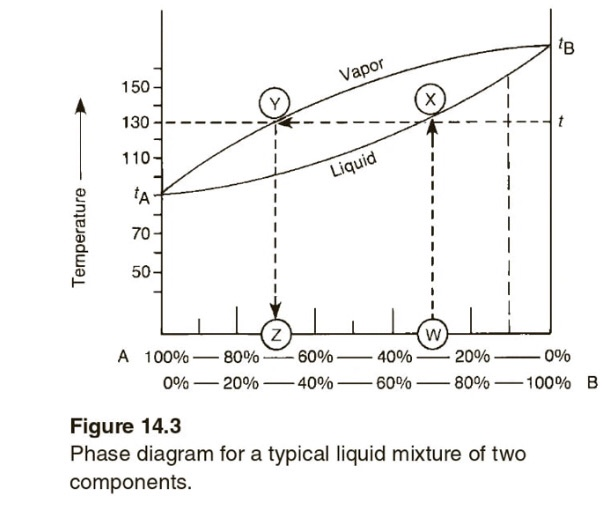
the vapor phase. C) A fractional distillation is, in effect, multiple distillations in the same setup. The first distillation is in the distillation flask. The other distillations are in the fractionating column. D) The GC detector responds differently to each compound. Thus, the areas of the peaks are not directly The figure shows the phase diagram of a system in which the liquids become fully miscible before they boil. Distillation of a mixture at a 1 leads to vapor with composition b 1, which condenses to completely miscible solution at b 2. Phase separation only occurs when the distillate is cooled to a point in the two-phase region such as point b 3. Thiele diagram, or the other methods developed for binary systems. This simplification can often be made when the amount of the non-key components is small, or where the components form near-ideal mixtures. Where the concentration of the non-keys is small, say less than 10%, they can be lumped in with the key components.
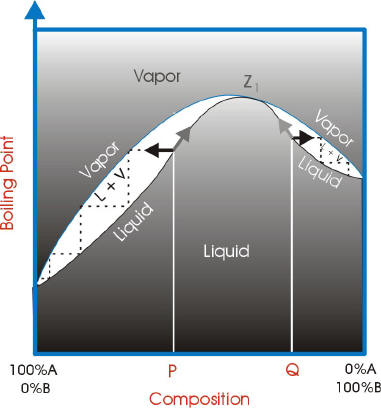
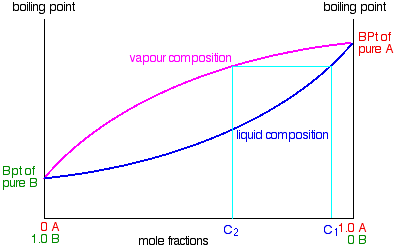
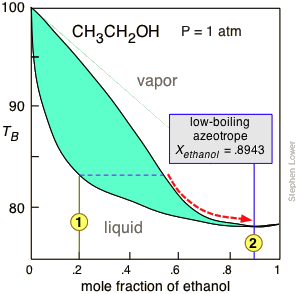
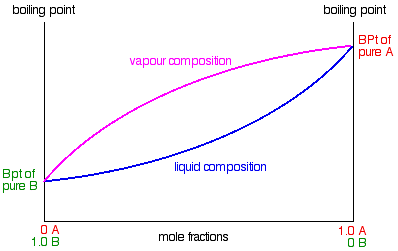
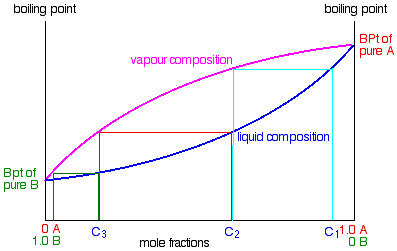
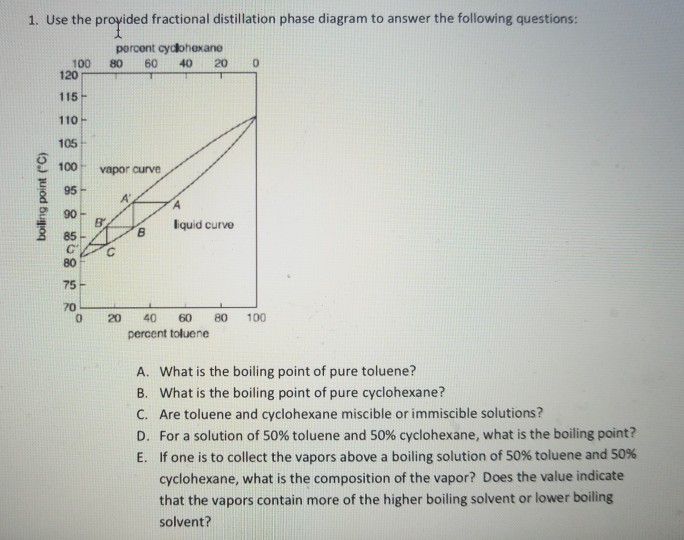
Fractional distillation phase diagram. Fig. 1: Distillation lines of an ideal ternary mixture (α1 =1,α2 =3,α3 =4) Eq. (11) can also be written in the form j =0 (13) which we call the Fundamental Equation of Distillation as the different methods of distillation like reversible distillation, simple distillation, distillation with theoretical trays and so on can be expressed in this ... To separate two volatile liquids with a phase diagram as shown in Fig. 2.7 fractional distillation is used, i.e. the boiling and condensation cycle is ... To understand the nature of simple distillation, fractional distillation and azeotropes we need to look at vapor/liquid diagrams for pairs of solvents. The graph below (Fig. 5) shows such a diagram for 2 solvents, A and B. A is the lower boiling material. The bottom of the graph shows the liquid state and the top of the graph shows the vapor state. This page explains how the fractional distillation (both in the lab and industrially) of an ideal mixture of liquids relates to their phase diagram. This is the second page in a sequence of three pages. Important: If you have come straight to this page from a search engine and are looking for simple ...
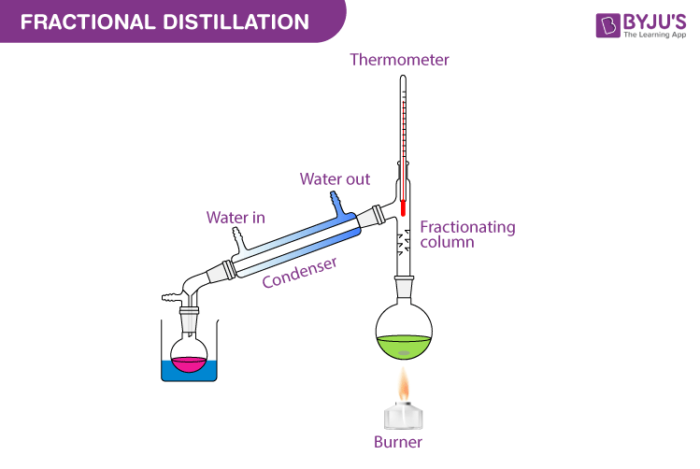
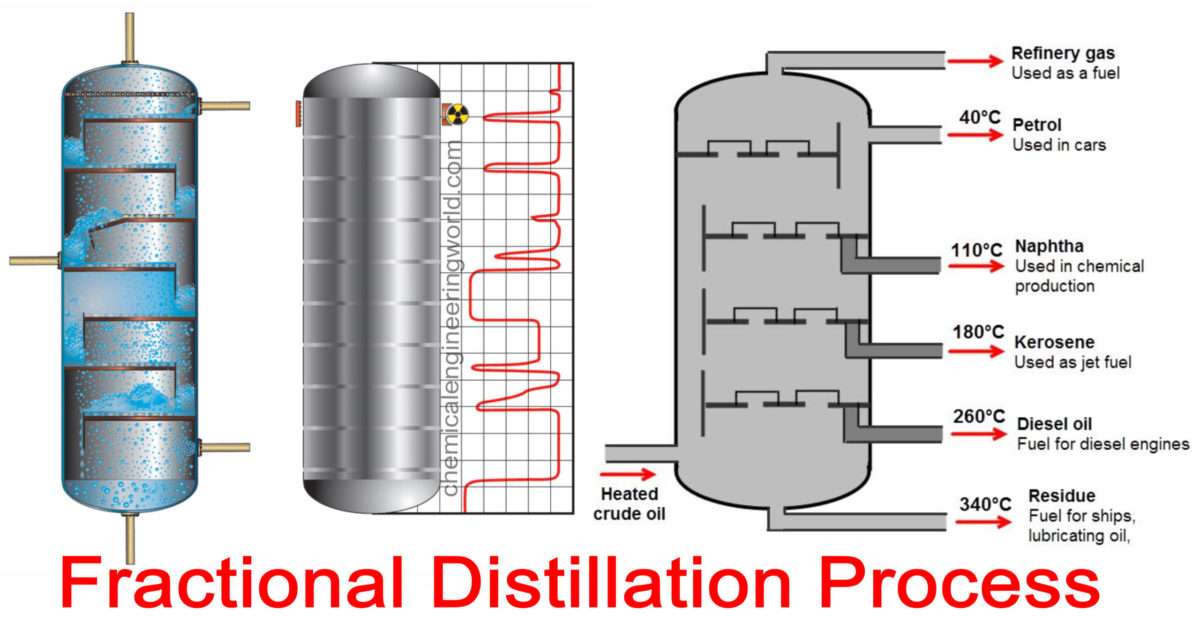
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a ... Using the phase diagram — On the last page, we looked at how the phase diagram for an ideal mixture of two liquids was built up.Using the phase diagram · Fractional Distillation in the lab · The vapor Fractional distillation is a type of distillation which involves the separation of miscible liquids. The process involves repeated distillations and condensations and the mixture is usually separated into component parts. The separation happens when the mixture is heated at a certain temperature where fractions of the mixture start to vaporize. Thiele diagram, or the other methods developed for binary systems. This simplification can often be made when the amount of the non-key components is small, or where the components form near-ideal mixtures. Where the concentration of the non-keys is small, say less than 10%, they can be lumped in with the key components.
The figure shows the phase diagram of a system in which the liquids become fully miscible before they boil. Distillation of a mixture at a 1 leads to vapor with composition b 1, which condenses to completely miscible solution at b 2. Phase separation only occurs when the distillate is cooled to a point in the two-phase region such as point b 3. the vapor phase. C) A fractional distillation is, in effect, multiple distillations in the same setup. The first distillation is in the distillation flask. The other distillations are in the fractionating column. D) The GC detector responds differently to each compound. Thus, the areas of the peaks are not directly

Consider The Phase Diagram Below For A Mixture Of Chloroform And Methanol Looking At How The Homeworklib

Phase Diagram Of The Vapor Liquid Liquid Solid Equilibrium Of The Water Nacl 1 Propanol System At 101 3 Kpa Sciencedirect











0 Response to "41 fractional distillation phase diagram"
Post a Comment