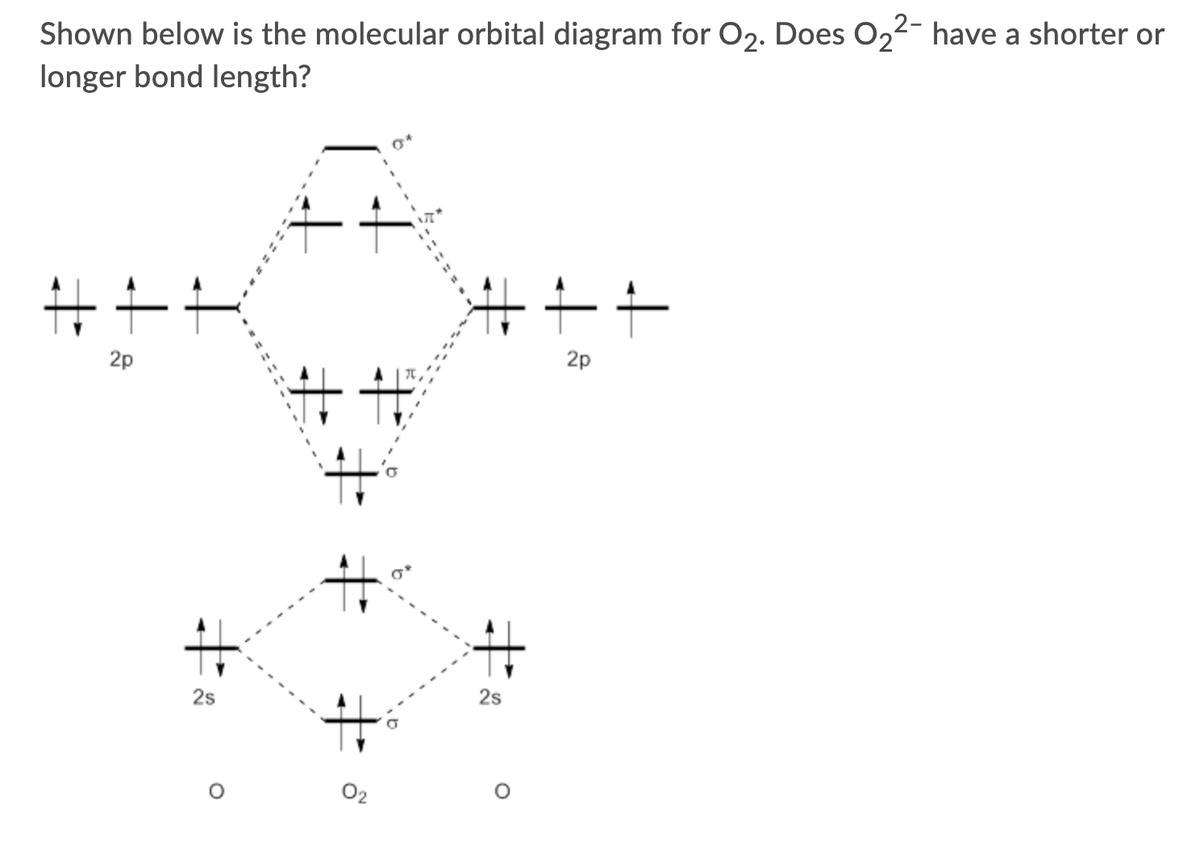
43 o22- molecular orbital diagram
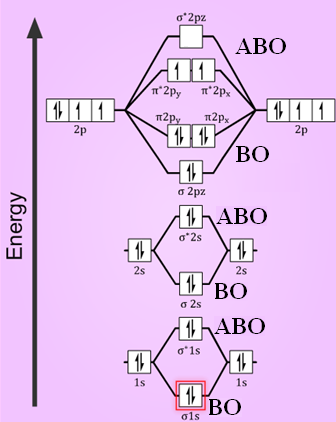
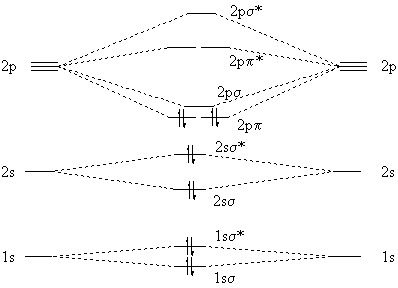
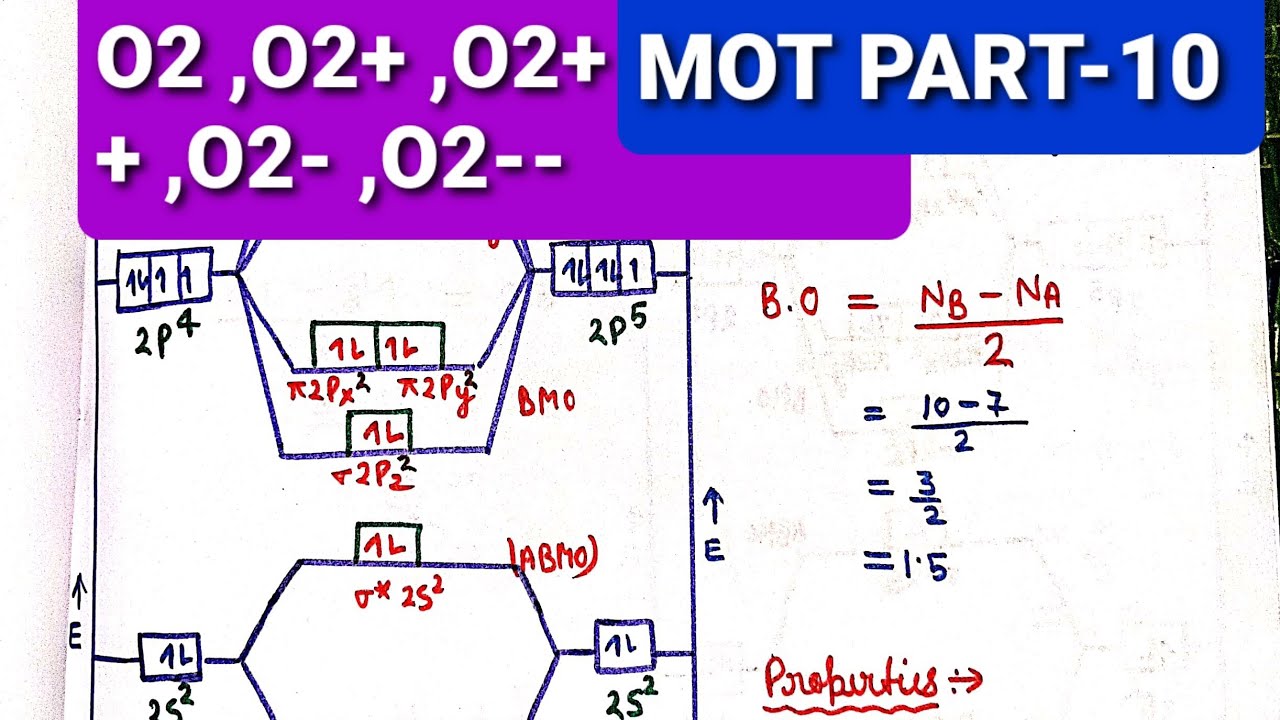
Follow me on instagram-https://www.instagram.com/trickychemistrysuman/?hl=enFollow me on facebook page-https://lm.facebook.com/l.php?u=https%3A%2F%2Ffb.me%2F... The molecular orbital energy level diagram of oxygen molecule is given as follows : Bond order 2 N b − N a = 2 8 − 4 = 2 Thus, oxygen molecule has two bonds. i.e., one is bond and one p bond.
Remember: When two oxygen atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals. They are flipped compare...
O22- molecular orbital diagram
Use the molecular orbital diagram shown to determine which of the following are paramagnetic. A o22 b ne22 c o22 d f22 e none of the above are paramagnetic. Perchlorates are powerful oxidizing agents used in. None of the above are paramagnetic. Home study science chemistry chemistry questions and answers draw the molecular orbital diagram shown ... Use the molecular orbital diagram shown to determine which of the following is paramagnetic. asked Jul 15, 2019 in Chemistry by brittanyr9777. general-chemistry. Nitrogen can lose an electron to form N2+. Given the molecular orbital configuration of N2 [core] (σ2s)2 (σ *2s)2 (π2p)4 (σ2p)2 is N2+ diamagnetic or paramagnetic? asked Jun 30 ... Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion. Recall that the formula for bond order is: Bond Order = 1 2 [ # of e - in bonding MO - # of e - in antibonding MO] 89% (181 ratings) Problem Details. Explain the following. The O22+ ion has a stronger O-O bond than O2 itself.
O22- molecular orbital diagram. According to molecular orbital theory the o22 molecular ion should be. Construct the molecular orbital diagram for he2 and then identify the bond order complete this valence molecular orbital diagram for oxygen o2. What is the molecular orbital electron configuration for o2 and how many unpaired electrons would it have. As it can be seen from the given structures that in the molecular orbital diagram for O 2 + ion, the highest occupied orbital is π ∗ MO orbital. Video Explanation Solve any question of Chemical Bonding and Molecular Structure with:- molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold rotation axis. The 2p and 2p * orbitals exhibit Cs symmetry. The latter do not possess C2 rotation axes coincident to the Answer (1 of 3): I modified the picture from this post: What's the MOT diagram of O2 +2 ion? and modified it to be O2 2+ (since sadly enough I am about as advanced with artistic programs on pc as a rock). How you basically do these questions is by first drawing the empty AO and MO, then counting ...
Oct 28, 2014 — Ans: The stabilities of these can be best explained using Molecular orbital theory. ... Atomic orbitals of oxygen combine to form molecular ... Molecular orbital diagram for o2 2. This ion has been observed in the gas phase. O2 molecular orbital diagram oxygen has a similar setup to h 2 but now we consider 2s and 2p orbitals. This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear ... Molecular orbital diagram for f2. In o 2 and f 2 there is a crossover of the sigma and the pi ortbials. Molecular orbitals mo are constructed from atomic orbitals. The relative energies of the sigma orbitals drop below that of the pi orbitals. The size of the effect depends on the 2s 2p energy difference. It is called a sigma molecular orbital ... The first photo is straight from a 2006 edition Pearson general chemistry textbook, and it shows you what the molecular orbital (MO) diagram for O2 is.5 answers · 31 votes: Hello! I actually just covered this question in my gen chem class this week. I have attached ...
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ... It is sigma2s(2)sigma2s*(2)sigma2p(2)pi2p(4)pi2p*(4)Bond order 1. It is stable. In fact, it's the perioxide ion.Check me out: http://www.chemistnate.com Atomic orbitals Molecular orbitals Atomic orbitals O, F, Ne Ne22 F₂2. F2 . 022- • F22 ; Question: Use the molecular orbital diagram shown to determine which of the following is most stable based on their bond order. Atomic orbitals Molecular orbitals Atomic orbitals O, F, Ne Ne22 F₂2. F2 . 022- • F22 Problem: Use the molecular orbital diagram shown to determine which of the following is most stable.a. F22+b. Ne22+c. F22-d. O22+e. F2
The valence electrons = 14; BO = 0.5* (8-6) = 1. The bond order is commonly used to signify the bond stability. Higher bond order indicates more stability and vice versa. Thus, is the most stable. is diamagnetic while and are paramagnetic in nature. Further Explanation: MO diagram: It is a tool used to describe the chemical bonding formed ...
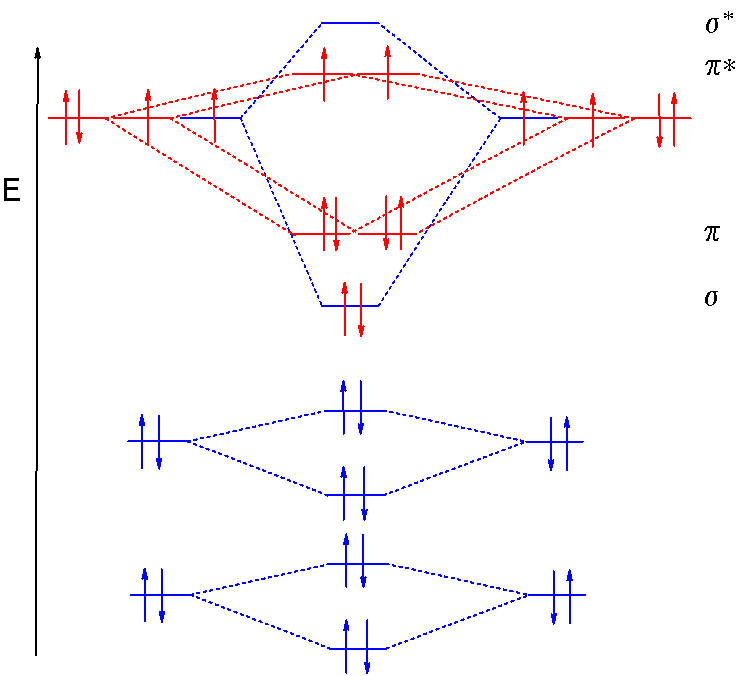
The atomic orbitals of proportional symmetry and comparable energy isO2+ > O2 > O2- > O22-so, we conclude is. D ) discuss the magnetic character of each molecule molecular orbital models of oxygen combine to form the molecular configuration! Orbitals combine to form molecular orbitals are formed by combination of atomic..
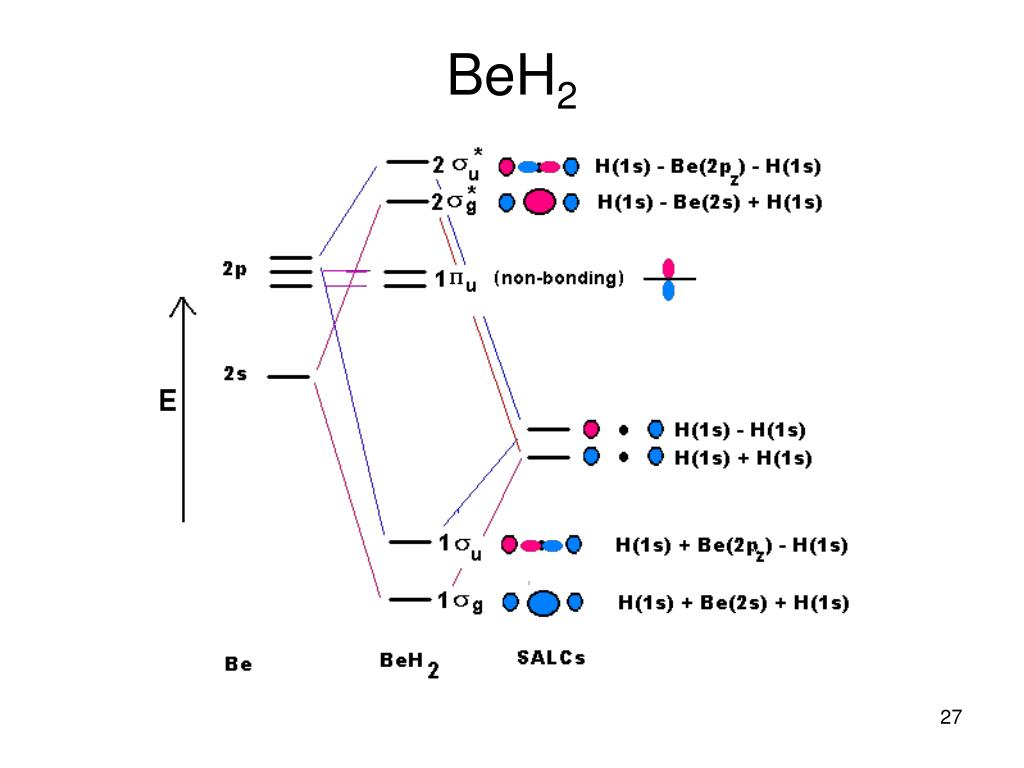
Ppt Ch2 Molecules And Covalent Bonding Lewis Structures Vsepr Mo Theory Powerpoint Presentation Id 6635712
Molecular Orbital Diagram Ne2 28.12.2018 28.12.2018 7 Comments on Molecular Orbital Diagram Ne2 Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.
How To make molecular Orbital diagramhttps://www.youtube.com/watch?v=UYC-ndQ6Lww&t=6s
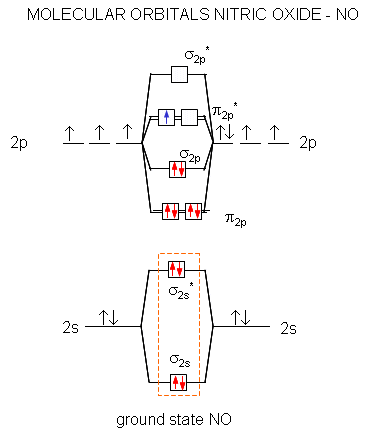
Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown:(i) Electronic configuration:(ii) Bond order: Here Nb = 8; Na = 4The two oxygen atoms in a molecule of oxygen are united through two covalent bonds ...
Problem: Draw molecular orbital diagrams for O2-, O22-, and O2. Which has the highest bond order? Which would be paramagnetic, and which would be diamagnetic? Can you draw good dot structures that correspond to each of these ions or molecules?
Hint: First draw a molecular orbital diagram (MOT) where the atomic orbitals combine to form molecular orbitals. The total electrons associated with the molecules are filled in the MOT diagram. To solve this question, we need to write the molecular orbital configuration. To find out the bond order from the molecular orbital configuration is:
Use molecular orbital diagram shown to determine which is most stable a) O22-b)F2 c) F22+ d) F22-e) Ne22-a. Use the molecular orbital diagram shown to determine which of the following is most stable. A) F2
Bond order (B.O) 1/2 × [Number of an electron in antibonding molecular orbitals] - [Number of electrons in bonding molecular orbitals] The higher the order of the bond the greater the pull between the two atoms and the shorter the length of the bond. (1) B.O for O 2 = 1/2 × [10 - 6] B.O for O 2 = 2
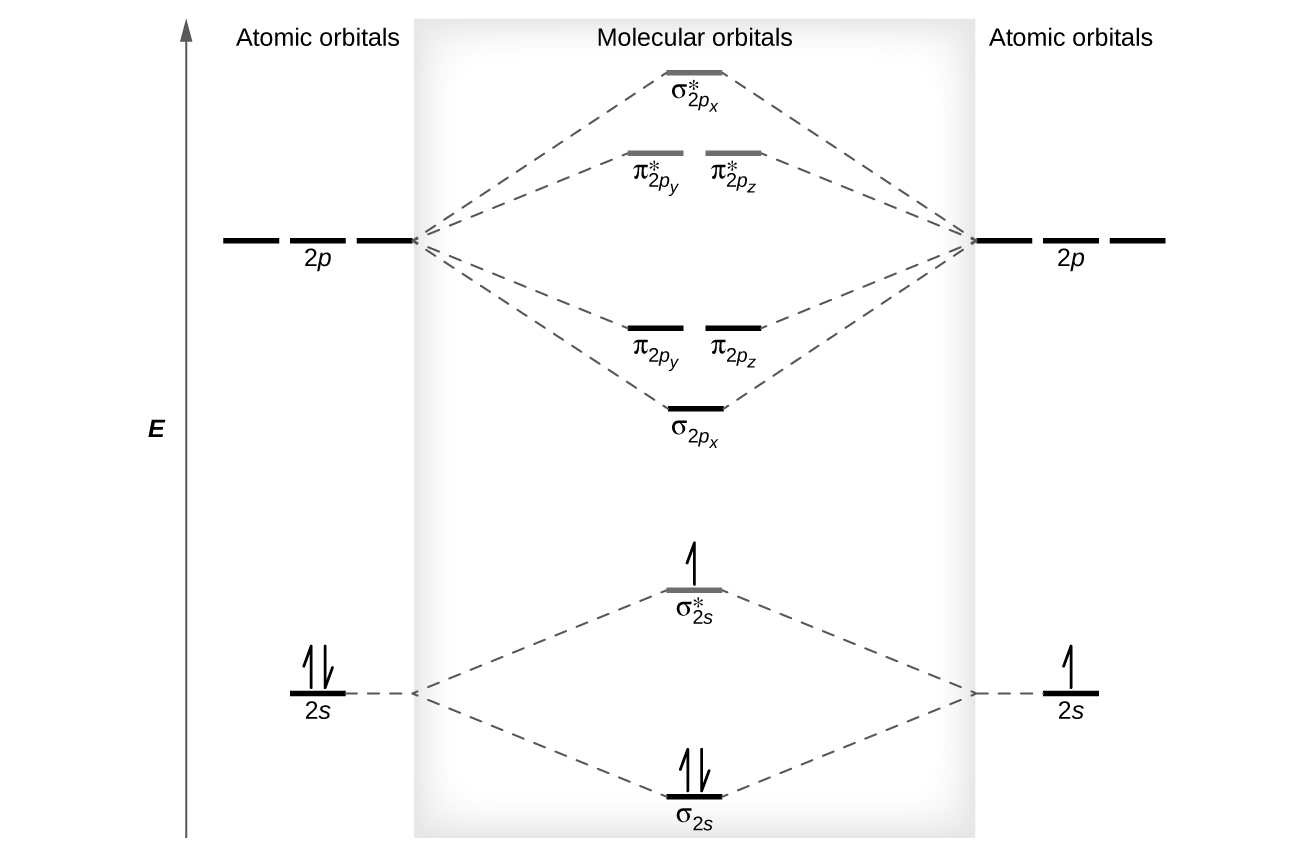
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
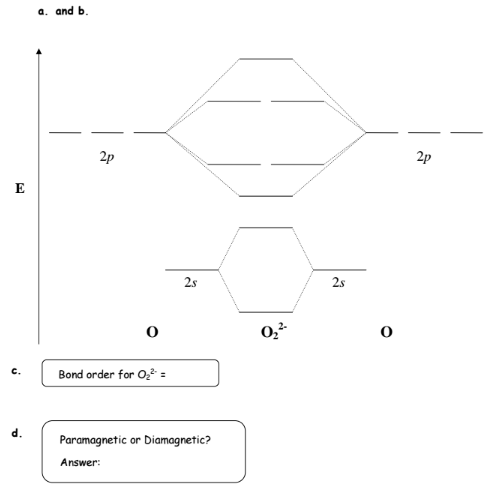
For the peroxide ion O22-:a) Draw the molecular orbital diagram.b) Calculate the bond order.c) Would this ion exist?d) Write the electron configuration of th...
1 answerDraw the MO diagram for O22- and identify the following: Bond order, diamagnetic or paramagnetic, homo and lumo. ... Hey there! We will begin by drawing the ...

Draw The Molecular Orbital Energy Diagram For Oxygen Molecule O2 And Show That I It Has A Double Bond Ii It Has Paramagnetic Character From Chemistry Chemical Bonding And Molecular Structure Class 11 Cbse
Draw molecular orbital diagrams for O2-, O22-, and O2. Which has the highest bond order? Which would be paramagnetic, and which would be diamagnetic? Can you draw good dot structures that correspond to each of these ions or molecules? Draw a molecular orbital diagram lor Arz*. This ion has been observed in the gas phase.
Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion. Recall that the formula for bond order is: Bond Order = 1 2 [ # of e - in bonding MO - # of e - in antibonding MO] 89% (181 ratings) Problem Details. Explain the following. The O22+ ion has a stronger O-O bond than O2 itself.

Q 17 What Is Meant By The Term Bond Order Mention The Bond Orders In O22 And Co Chemistry Chemical Bonding And Molecular Structure 11716277 Meritnation Com
Use the molecular orbital diagram shown to determine which of the following is paramagnetic. asked Jul 15, 2019 in Chemistry by brittanyr9777. general-chemistry. Nitrogen can lose an electron to form N2+. Given the molecular orbital configuration of N2 [core] (σ2s)2 (σ *2s)2 (π2p)4 (σ2p)2 is N2+ diamagnetic or paramagnetic? asked Jun 30 ...

Molecular Orbital Theory Homodiatomics Use The Molecular Orbital Model To Fully Describe The Bonding In O2 O2 O2 And O22 Determine Which Of The Following Statements Are True And Which Are
Use the molecular orbital diagram shown to determine which of the following are paramagnetic. A o22 b ne22 c o22 d f22 e none of the above are paramagnetic. Perchlorates are powerful oxidizing agents used in. None of the above are paramagnetic. Home study science chemistry chemistry questions and answers draw the molecular orbital diagram shown ...
Molecular Orbital Theory Chemistry Encyclopedia Structure Number Molecule Atom Bond Order Multiple Bonds
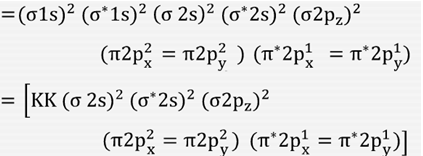


















0 Response to "43 o22- molecular orbital diagram"
Post a Comment