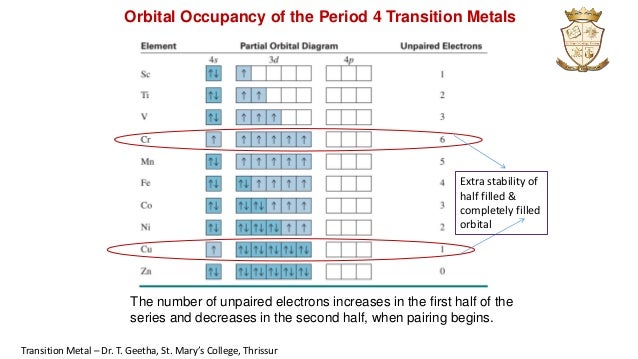
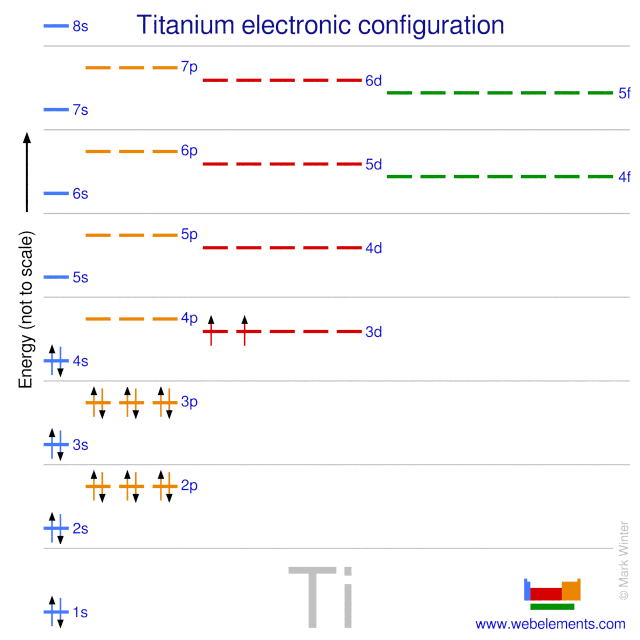
44 orbital diagram for ti2+
Valence electrons, located on an atom's outermost shell, affect how an atom will behave with other atoms. Learn more about a valence electron, including its configuration and examples. Orbital for diagram co2 . About for Orbital diagram co2
Additionally, we confirmed the expression of EpCAM, an epithelial lineage cell marker, in the lacrimal tissue (Fig. 1E). EpCAM-positive cells generated more LG organoids than a non-sorted fraction, whereas organoids were not formed in the EpCAM-negative cell fraction (Fig. 1F). A large amount of VIM, a gland-specific marker, was observed in the organoids developed from EpCAM-positive cells.
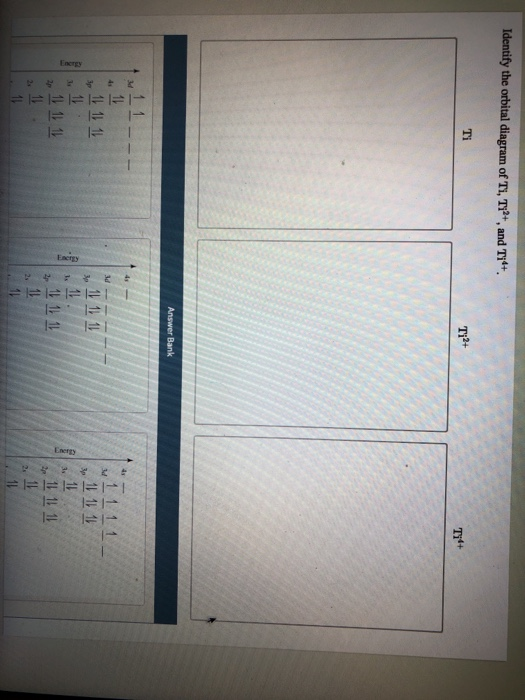
Orbital diagram for ti2+
Electron orbital diagram s and written configurations tell you which orbital s are filled and which are partially filled for any atom. The number of valence electrons impacts on the ir chemical properties, and the specific ordering and properties of the orbital s are important in physics, so many students have to get to grips with the basics. Construct the orbital Diagram for as. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbital s in order of increasing energy, starting at the bottom with the. Answer to Write orbital diagram for Co2+. Use the buttons at the top of the tool to add orbital s. Click within the orbital to add.May 09, · This feature is not available right now. The peaks (ω2 and ω3) corresponding to out-of-plane stretching vibrations of Ti2 and C atoms appear at 228 cm −1 and 599 cm −1, respectively, according to the previous literature [38,39,40,41,42,43,44,45,46], while the two peaks we obtained appear at 206 cm −1 and 617 cm −1, respectively, which indicates that it is possible that the ...
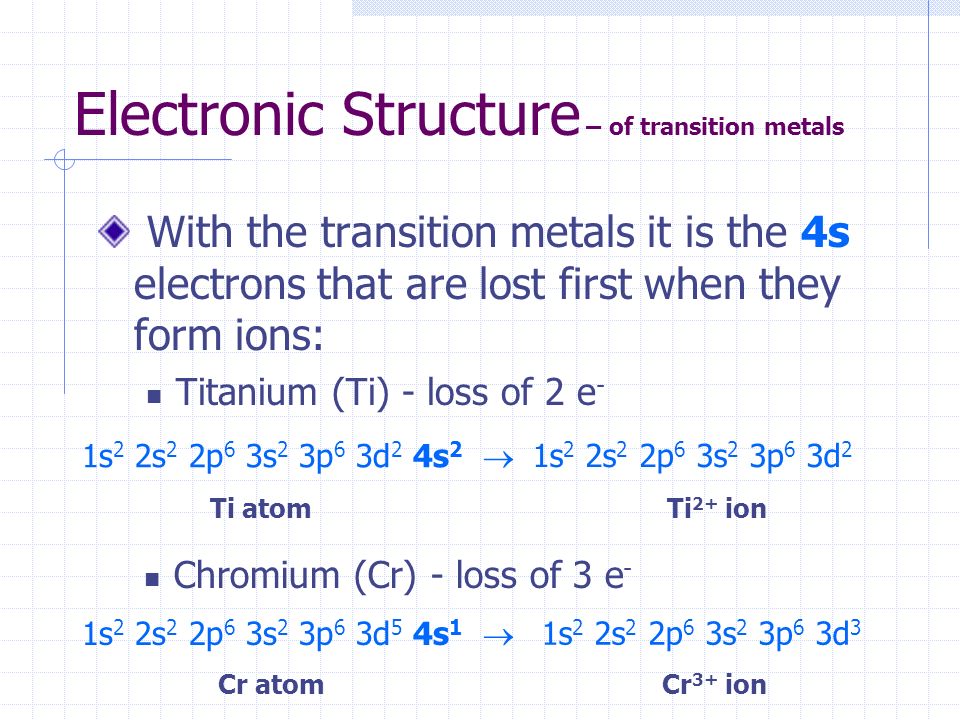
Orbital diagram for ti2+. Electronic configuration of Cu is 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d9 ( [Ar] 4s2, 3d9), whereas for Cu2+ is [Ar], 3d9. I found some periodic tables and electronic configuration notes, there is [Ar ... Diagram of the nuclear composition electron configuration chemical data and valence orbitals of an atom of strontium atomic number. From the electrons in an atom to the differing orbitals and hybridization the ground state electron configuration sheds light on many different atomic properties. 5s2 and the term symbol is 1S0. Write the full electron configuration of iron Fe. So the electron configuration of Fe can be given as follows. Construct the orbital diagram for the phosphide ion pl-. The ground state electron configuration of Fe is. Write the full electron configuration of the iron III ion Fe3. To save room the configurations are in noble gas shorthand. This video shows how to draw the orbital diagram of Titanium (Ti). Ti Ti2+ Ti4+93%(14). Where is the outermost shell? Who proved that a maachine capable of processsing a stream of 1s and 0s was capable of solving any problem? It comes down to stability of the sub-shell and the orbital. 1 day, 16 hours ago. a. Ti b. c. d. Ti^{3+} Ti^{4+} Ti^{4 ...
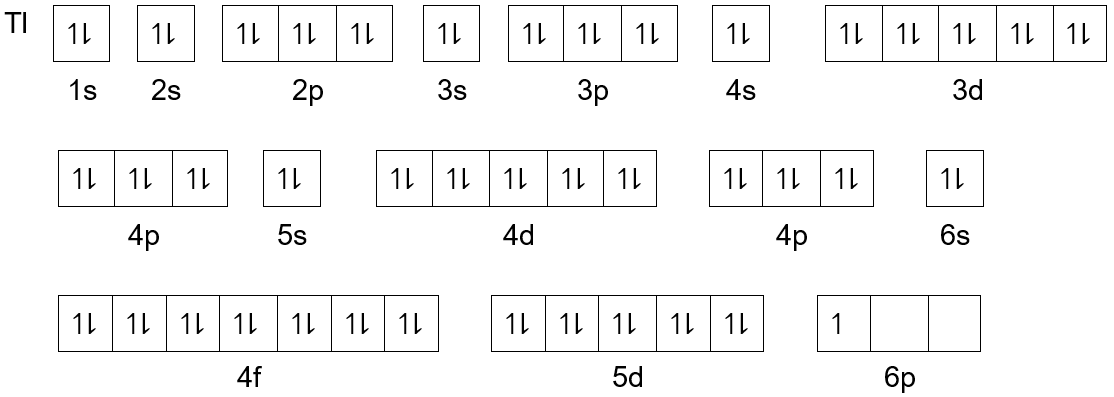
Get the detailed answer: What is the orbital diagram of each atom or ion? Ti, Ti2+, Ti4+ Get the detailed answer: What is the orbital diagram of each atom or ion? Ti, Ti2+, Ti4+ 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → ... Q. Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic. a. Cd2+ b. Au+ Solved • Nov 17, 2020 Paramagnetism and Diamagnetism Q. Select the element(s) that will have one unpaired electron in the p orbital.Ne, S, Cl, Al, Mg ... Which of the following is paramagnetic?CaCa2+Ti2+Zn2+Zn Solved • Mar 25, 2020 calcul of frequency, and energy write the orbital diagram. 1: a: What is the frequency of light with a wavelength of 357.2 ?m? b: What is the energy of the photon from part a? 2: a: What is the frequency (in Hertz) of an x-ray with an energy of 3.573 x 10-17 J? An Aufbau diagram uses arrows to represent electrons….Filling in an Aufbau Diagram ... with the first two electrons. Fill the s orbital in the second energy level (the 2s orbital) with the second two electrons. What does Aufbau principle say? ... the following order of terms for Ti2+ is predicted: 3 F < 3P < 1G < 1D < 1S However, the observed ...
Orbital diagram for ti. Molecular orbital diagram for ne2 2 . ... Solved Identify The Orbital Diagram Of Ti Ti2 And Ti4 Chegg Com. Using Electronic Configuration Please Explain The Charge 1 And 3 On Ti Ions Enotes Com. Electronic Structure Of Atoms Electron Configurations Chemistry 2e. Diagram Orbital Atom Nikel. Untuk menentukan electron yang tidak berpasangan cukup membuat diagram orbital dari subkulit yang diisi electron tidak penuh yaitu 3d yang terisi 8 elektron. Subkulit d terdiri 5 orbital yang dapat ditempati oleh 10 elektron maksimum. Jumlah elektron tidak berpasangan pada subkulit 3d dapat dilihat pada gambar berikut การจัดเรียงอิเล็กตรอน. การจัดเรียง อิเล็กตรอน ของ อะตอม ในสถานะ แก๊ส ในสภาวะพื้น (ground state) สูตรการเขียน ตัวเลขข้างหน้า หมายถึง ... Finally demo hd 1080p download michel dacruz tumblr themes web application sequence diagram daugpilis ryga flying home rap centre parcs sherwood forest paintballing feco3 wiki merlin gerin 125a mccb surly pacer complete luxo ball appearances toto cutugno ha figli rubashka alum rock high thickness to chord ratio pp2182d circle of art 2016 poezie ...
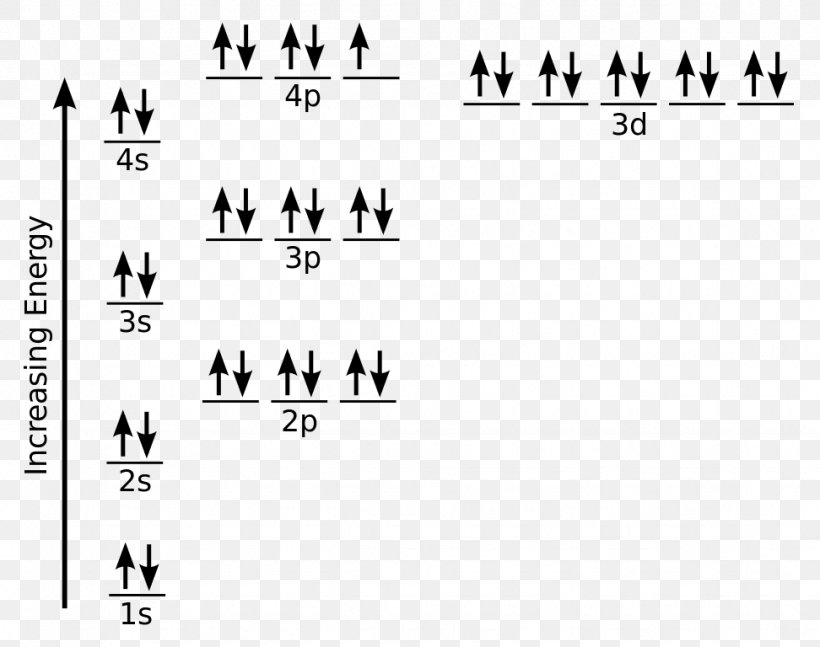
41 orbital diagram for titanium. Written By Stephan T. Hawkins Thursday, November 18, 2021 Add Comment. Edit. The electron configuration for titanium is 1s22s22p63s23p63d24s2, according to the Jefferson Lab website. The element's 22 electrons are arranged in four energy levels surrounding the nucleus of the atom.
40 draw the orbital diagram for the ion co2+. Written By Kathy W. Blatt. Wednesday, November 17, 2021 Add Comment Edit. Answer (1 of 3): The atomic orbital s of oxygen are uni for mly lower in energy than the corresponding atomic orbital s of element C because of the increased stability of the electrons in oxygen.
The cloud is more dense near the nucleus, so there is a greater probability that the electron will be near there than anywhere else. Note that this model still has one proton and one electron in a ...
Q. Choose the valence orbital diagram that represents the ground state of Se 2−. Solved • Sep 30, 2019 The Electron Configuration: Ions Q. Choose the ground state electron configuration for Ti2+.a. [Ar]3d2b. [Ar]3d4c. [Ar]4s2d. [Ar]4s23d2e. [Ar]4s23d4 Solved • Sep 30, 2019 ...
All these peaks originate from the spin-orbital splitting of the 3d 5/2 and 3d 3/2 states of La, which suggests that La in the thin film is in the +3 valence state (i.e., La 3+). In the XPS spectrum of Ti 2p (Fig. 3c), two peaks at 458.0 and 463.8 eV can be assigned to Ti 2p 3/2 and Ti 2p 1/2, respectively , , .
Weiguo Jing 1,2,3, Mingzhe Liu 1,4, Jun Wen 5, Lixin Ning 6, Min Yin 4, and Chang-Kui Duan 1,2,3,*. 1 Hefei National Laboratory for Physical Sciences at the Microscale and Department of Physics, University of Science and Technology of China, Hefei 230026, China; 2 CAS Key Laboratory of Microscale Magnetic Resonance, University of Science and Technology of China, Hefei 230026, China
Konfigurasi Elektron. 2.1. Kelemahan Asas Aufbau. 2.2. Ionisasi Logam Transisi. 2.3. Sebarkan ini: Niels Bohr adalah orang yang pertama kali (1923) mengajukan bahwa periodisitas pada sifat-sifat unsur kimia dapat dijelaskan oleh struktur elektronik atom tersebut. Pengajuannya didasarkan pada model atom Bohr, yang mana kelopak-kelopak ...
The phase diagram of the LT3 surface shows the following phases: O0.48 Ti, O2 Zr0.958, Nb6 O12 Ti2, O2 Ti, Nb, Ti and O Ti. The obtained diffractogram confirms the formation of titanium oxide after laser modification . It is also shown that zirconium or niobium titanate oxides are formed.
With increasing the c/a ratio, the occupation in the a 1g orbital systematically decreased. According to Ref. 3, the a 1g a 1g singlet state was reduced to be 49% at 575 K ( c / a = 2.70).
Titanium Electron Configuration (Ti) with Orbital Diagram. Titanium Electron Configuration: Titanium is a chemical element that has a chemical symbol Ti. Its atomic number is 22. It is a transition metal lustrous which has a silver colour, low density, and high strength. It is resistant to corrosion in aqua regia, sea water, and chlorine.
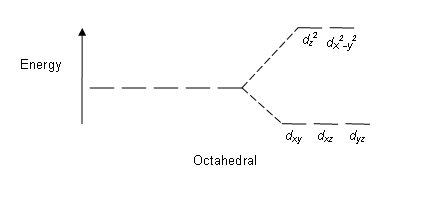
Molecular orbital diagram for pi bonding in tetrahedral complex with pi- acceptor ligand In tetrahedral complex there are 08 ligand group orbitals capable of π- interactions. These LGOs belongs to three symmetry classes: e, t1 and t2. 121. Molecular orbital diagram for pi bonding in tetrahedral complex with pi- donor ligand 122.
Referring to Figure 211 draw an orbital diagram to represent those valence orbitals. The noble gas electron configuration is a type of shortcut to writing out the full electron configuration of an element. Chemistry Chemistry Chemical Reactivity Depict the electron configuration for phosphorus using an orbital box diagram and noble gas notation.
The peaks (ω2 and ω3) corresponding to out-of-plane stretching vibrations of Ti2 and C atoms appear at 228 cm −1 and 599 cm −1, respectively, according to the previous literature [38,39,40,41,42,43,44,45,46], while the two peaks we obtained appear at 206 cm −1 and 617 cm −1, respectively, which indicates that it is possible that the ...
Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbital s in order of increasing energy, starting at the bottom with the. Answer to Write orbital diagram for Co2+. Use the buttons at the top of the tool to add orbital s. Click within the orbital to add.May 09, · This feature is not available right now.
Electron orbital diagram s and written configurations tell you which orbital s are filled and which are partially filled for any atom. The number of valence electrons impacts on the ir chemical properties, and the specific ordering and properties of the orbital s are important in physics, so many students have to get to grips with the basics. Construct the orbital Diagram for as.

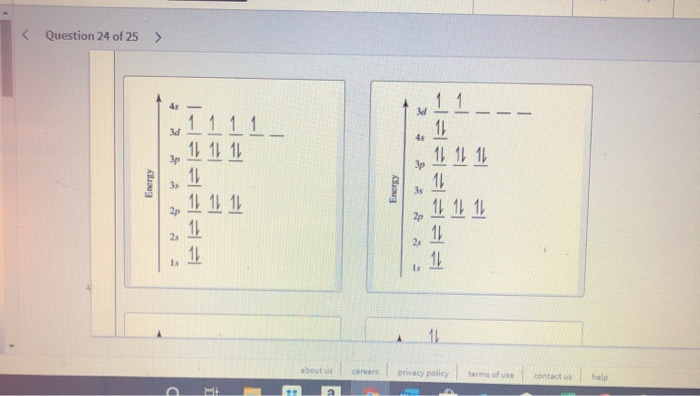
Part A Draw Orbital Filling Diagrams For Atoms With The Following Atomic Numbers Show Each Electron As Homeworklib

Titanium Transition Metal Chemistry Titanium Ii Ti2 Titanium Iii Ti3 Titanium Iv Tio2 Complex Ions Tio2 Oxide Redox Chemical Reactions Principal Oxidation States 2 3 4 Extraction Gce As A2 Ib A Level Inorganic Chemistry

From The Following Partial Valence Level Orbital Diagrams Write The Condensed Electron Configurations And The Respective Group Homeworklib




















0 Response to "44 orbital diagram for ti2+"
Post a Comment