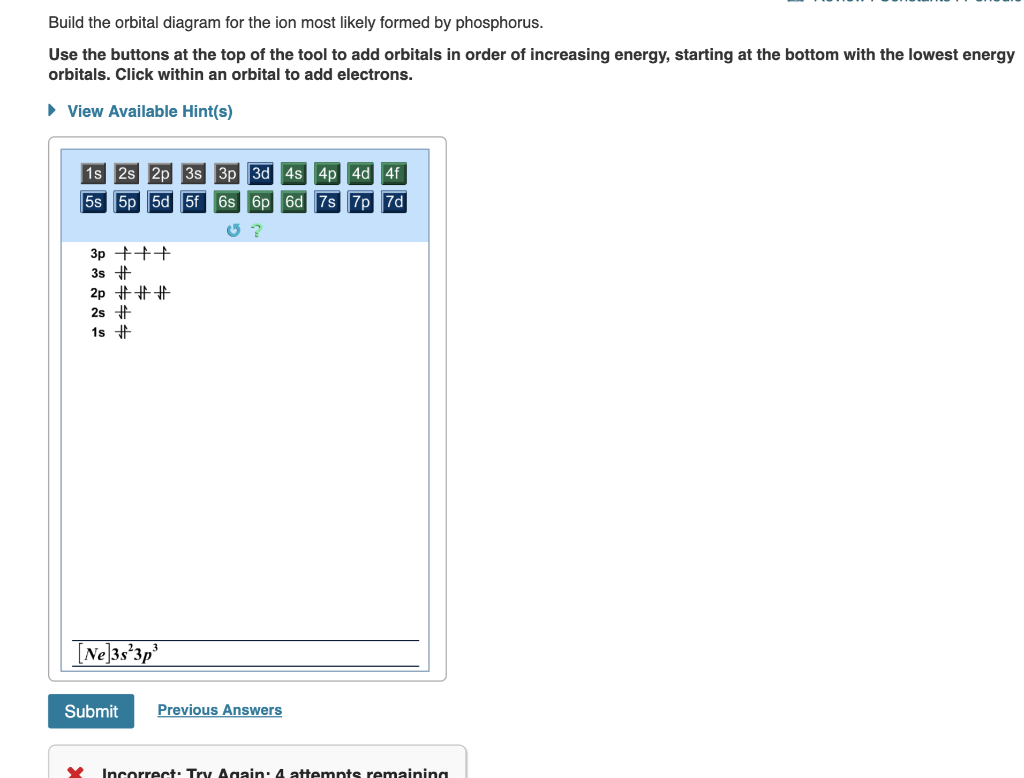
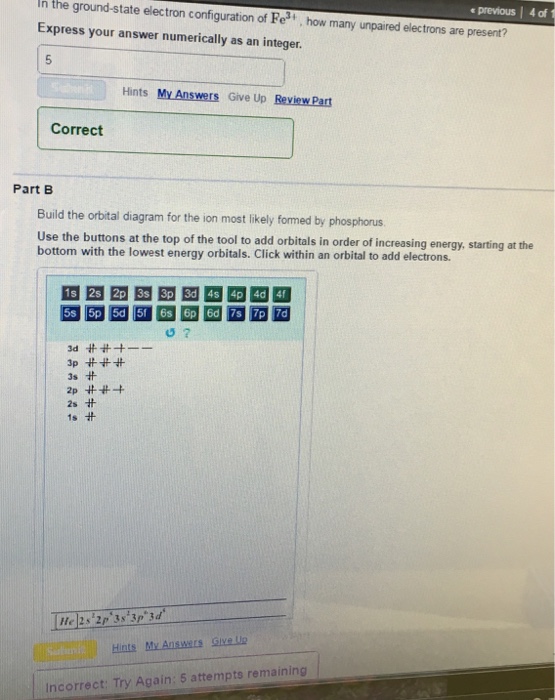
39 build the orbital diagram for the ion most likely formed by phosphorus.
A chlorine ion has 18 electrons 17 e- in a chlorine atom 1 e- to form the chlorine anion having -1 charge. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic structure. In this video we will write the electron configuration for Cl- the Chloride ion. M shell 7 electrons.
Answer (1 of 1): The orbital notation for Phosphorus is: Arrow pointing up, arrow pointing down, for one line. Sodium, which symbolized as Na is a group 1 element with the atomic number 11. S -2 and K1+ ions. Start studying Orbital Notation + Electron Configuration.
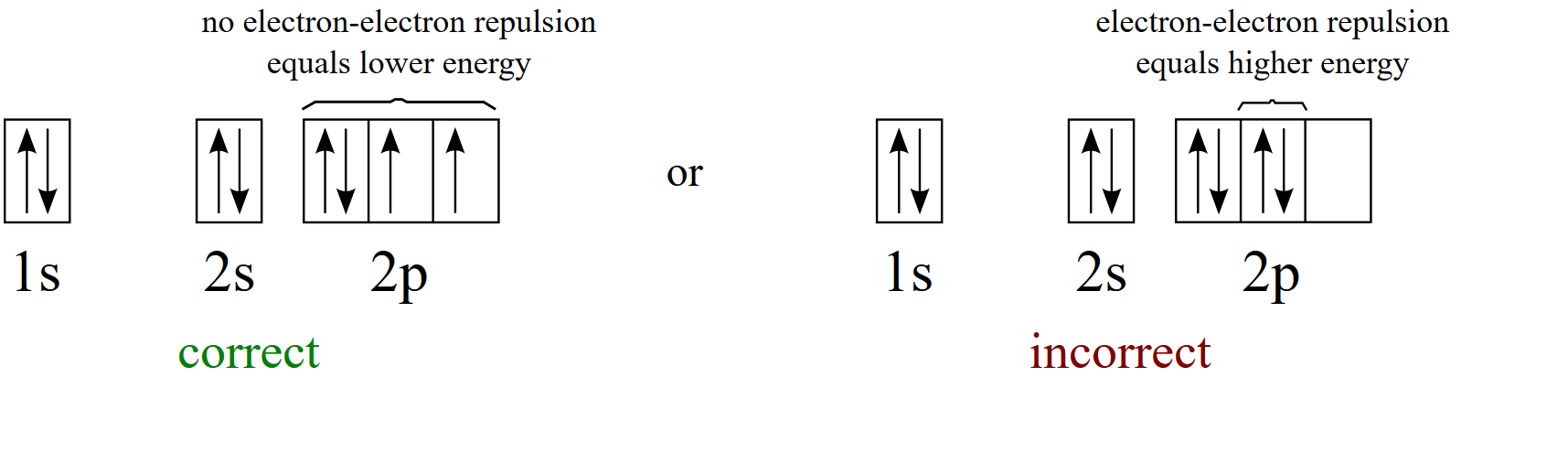
Orbital diagrams are pictorial descriptions of the electrons in an atom. Three guidelines are helpful in forming orbital diagrams. In accordance with the Auf Bau Precept, every electron occupies the bottom vitality orbital. You bounce up just a little bit in vitality and we get the 2s orbital that make it the 2p sublevel.
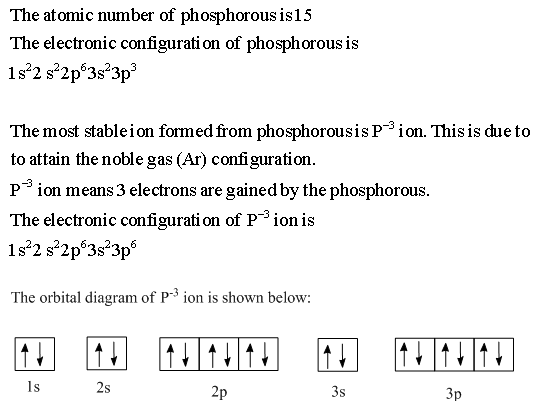
Build the orbital diagram for the ion most likely formed by phosphorus.
Valence electrons can greatly impact the properties of atoms of the same element. The electron is one of the most important factors in determining how an atom will react with another atom or ...
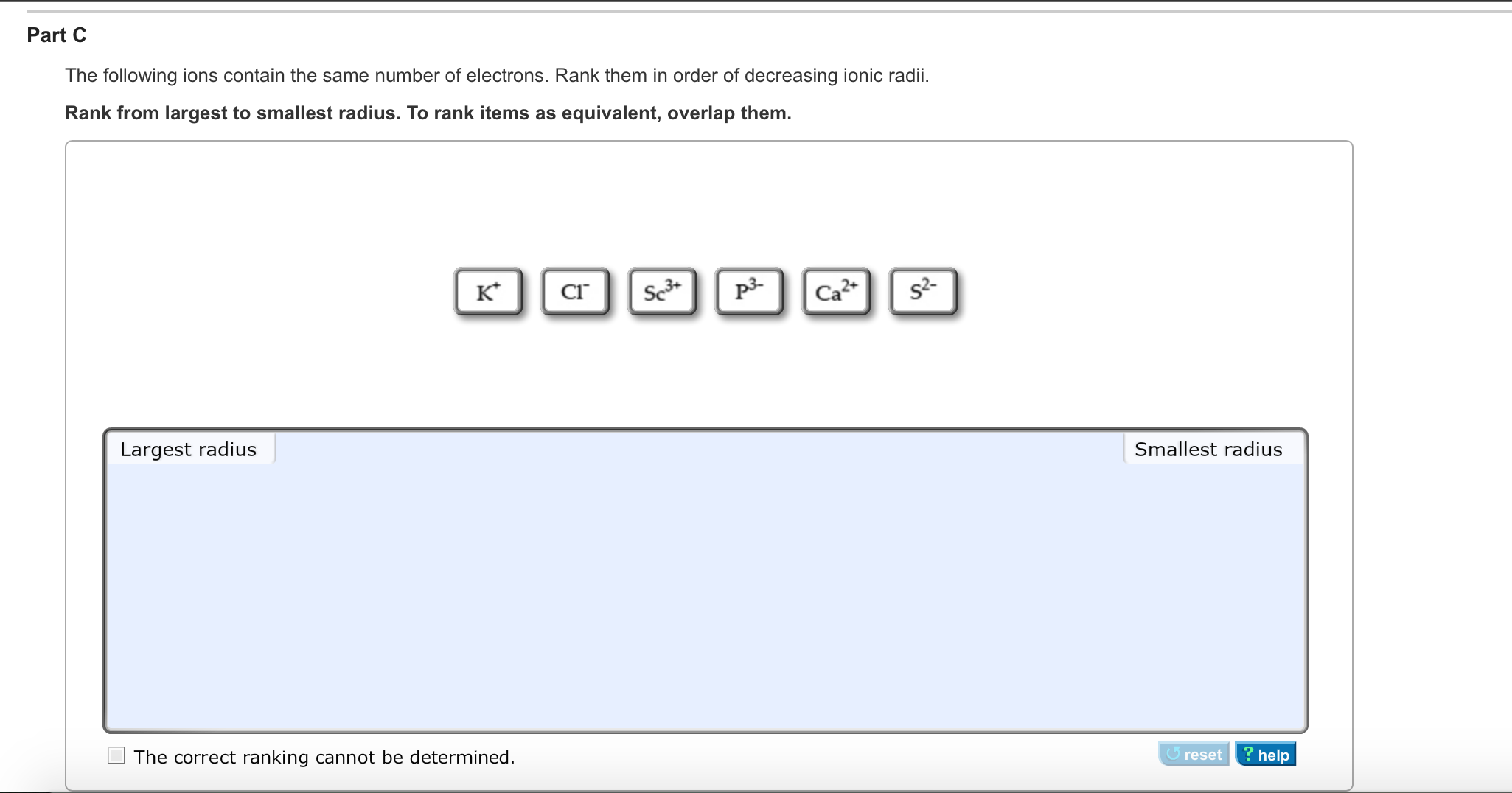
Electron Configurations of lons ③ 19 01 23 Review Constants Periodic Table When an atom forms an ion, it will gain or lose electrons to attain a more stable electron configuration, frequently that of a noblo gas. Nonmetals tend to form anions by gaining electrons, which enter the lowest energy unoccupied orbital.
Start studying Orbital Diagrams, Electron Configuration, Valence electrons. ... The arrows represent the 16 electrons of the sulfur atom and the directions .... The Bohr model was a one-dimensional model that used one quantum number to describe the distribution of electrons in the atom.
Build the orbital diagram for the ion most likely formed by phosphorus..
Phosphine or AKA Phosphorus Tri-hydrate (PH3) is the most misunderstood chemical compound in chemistry and the reason is it's a polar molecule with non-polar bonds. Hence, the compound is crushed to the core and gains the ability to heat a talk to a debate. Its pure form is odorless, and other forms have an unpleasant odor like a rotten fish ...
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. When electrons are in an orbital its a pair and when they are furthest away they repel each other most and are most likely to be flung off. 119 Zeilen The electronic configuration of each element is decided by the ...
The Aufbau Principle. We construct the periodic table by following the aufbau principle (from German, meaning "building up"). First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli principle.We use the orbital energy diagram of Figure \(\PageIndex{1}\), recognizing that each orbital can ...
When it forms ion it will lose 3 electrons 2 from 4S and 1 from 3d. - can be written using the period table or an electron configuration chart. Build the orbital diagram for the ion most likely formed by phosphorusin the ground state electron configuration of rm fe 3 how many unpaired electrons are presenthow many valence electrons does a tin.
From the perspective of applications and chemical literature, the most important form of elemental phosphorus is white phosphorus, often abbreviated as WP.It is a soft, waxy solid which consists of tetrahedral P 4 molecules, in which each atom is bound to the other three atoms by a single bond. This P 4 tetrahedron is also present in liquid and gaseous phosphorus up to the temperature of 800 ...
Diagram of the nuclear composition and electron configuration of an atom of phosphorus-31 (atomic quantity: 15), the most typical isotope of this factor. The nucleus consists of 15 protons (crimson) and 16 neutrons (blue). 15 electrons (inexperienced) bind to the nucleus, successively occupying out there electron shells (rings).
Referring to either Figure 6.4. 3 or 6.4. 4, we would expect to find the electron in the 1 s orbital. By convention, the m s = + 1 2 value is usually filled first. The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2.
Now we know that Bromine has 35 electrons. P-orbital can take maximum 6 electrons. Well also look at why Bromine forms a 1- ion and how the electron configura. Nevertheless check the complete configuration and other interesting facts about Bromine that most people dont know. Ive drawn the overlaps below in the MO diagrams.
PBr5 Molecular Geometry, Lewis structure, Shape, Bond Angle, And More. July 23, 2021. Posted by Priyanka. 10 Jun. Phosphorus pentabromide written as PBr5 in the chemistry equations is a reactive yellow solid. The compound has one molecule of Phosphorus and five Bromine molecules. Bromine is a halogen from Group 17 of the periodic table.
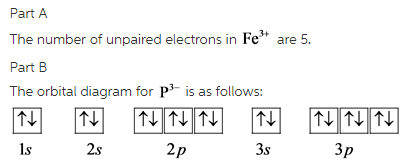
Orbital diagram for the ion most likely formed by phosphorus. Asked by wiki @ 12/06/2021 in Chemistry viewed by 55 persons. Enter the electron configuration for the ion most likely formed by phosphorus. Express your answer in the order of orbital filling as a string without blank space between orbitals. For …
30 Build The Orbital Diagram For The Ion Most Likely ... Part B Build the orbital diagram for the ion most likely ... 34 Build The Orbital Diagram For The Ion Most Likely ... High School Chemistry/Orbital Configurations - Wikibooks ... 8.4 Molecular Orbital Theory - Chemistry Orbital Box Diagram Phosphorus Aufbau Diagram For Phosphorus
Here, one 's' electron goes to the d orbital in excited form. Therefore, the one 3s electron and the other three electrons in 3p orbitals combine together to result in sp3. Conclusion. Here, in this article, we discussed in detail one of the most prominent halides of phosphorus.
Box spin diagram of outer electron orbitals for the electron configuration of the 15 Phosphorus P 1s22s22p63s23p3 Ne3s 3p P pblock Gp5 Build the orbital diagram for the ion most likely formed by phosphorus. The rules for orbital filling diagrams. Energy 0 1 1 x 5. Now this is only one way we can draw the electron dot diagram for Oxygen. Draw ...
the CO3^2- ion exists in only one form: an average of the three principal structures shown. Which concepts describes the formation of four equivalent, single, covalent bonds by carbon in its compounds that resembles methane, CH4
build the orbital diagram for the ion most likely formed by phosphorus. asked Aug 25, 2020 in Other by megha00 Expert (28.8k points) 0 votes. 1 answer. Quick lime and phosphorus pentaoxide cannot be used for drying hydrochloric acid gas. asked Jan 28, 2019 in Class X Science by aditya23 Expert (73.6k points)
Question: Build the orbital diagram for the ion most likely formed by phosphorus. This problem has been solved! See the answerSee the answer ...
Enter the electron configuration for the ion most likely formed by phosphor 1s^2 2s^2 2p^6 3s^2 3p^3, -3 to make stable so its 1s^2 2s^2 2p^6 3s^2 3p^6 The electron-electron repulsion due to pairing of electrons in oxygen makes its first ionization energy --- than expected.
Transcribed image text: Build the orbital diagram for the ion most likely formed by phosphorus. Use the buttons at the top of the tool to add sublevels.
build the orbital diagram for the ion most likely formed by phosphorus. asked Aug 25, 2020 in Other by megha00 Expert (39.0k points) 0 votes. 1 answer. What is the hybridization of phosphorus in PCl3 ? asked Dec 20, 2019 in Important Questions by megha00 Expert (39.0k points) Categories. All categories;
Transcribed image text: Build the orbital diagram for the ion most likely formed by phosphorus. Use the buttons at the top of the tool to add orbitals in ...
How many unpaired electrons are contained in each element: (a) chlorine, (b) selenium, (c) cesium, and (d) phosphorus? build the orbital diagram for the ion most likely formed by phosphorus.,in the ground state electron configuration of \rm fe 3+ how many unpaired electrons ,are present,how many valence electrons does a tin (sn) atom have ...
Transcribed image text: Build the orbital diagram for the ion most likely formed by phosphorus. Use the buttons at the top of the tool to add orbitals in ...
Build the orbital diagram for the ion most likely formed by phosphorus. science A chemistry class is experimenting with iodine to see which common white powder items will react and make the iodine change color.
Transcribed image text: Part BBuild the orbital diagram for the ion most likely formed by phosphorus. Use the buttons at the top of the tool to add orbitals ...
Ion electron confugurat ion s. A neutral fluorine atom has 9 electrons. Show transcribed image text construct the orbital diagram of the f ion. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbital s in order of increasing energy, starting at the bottom with the. Answer to Write orbital diagram for Co2+.
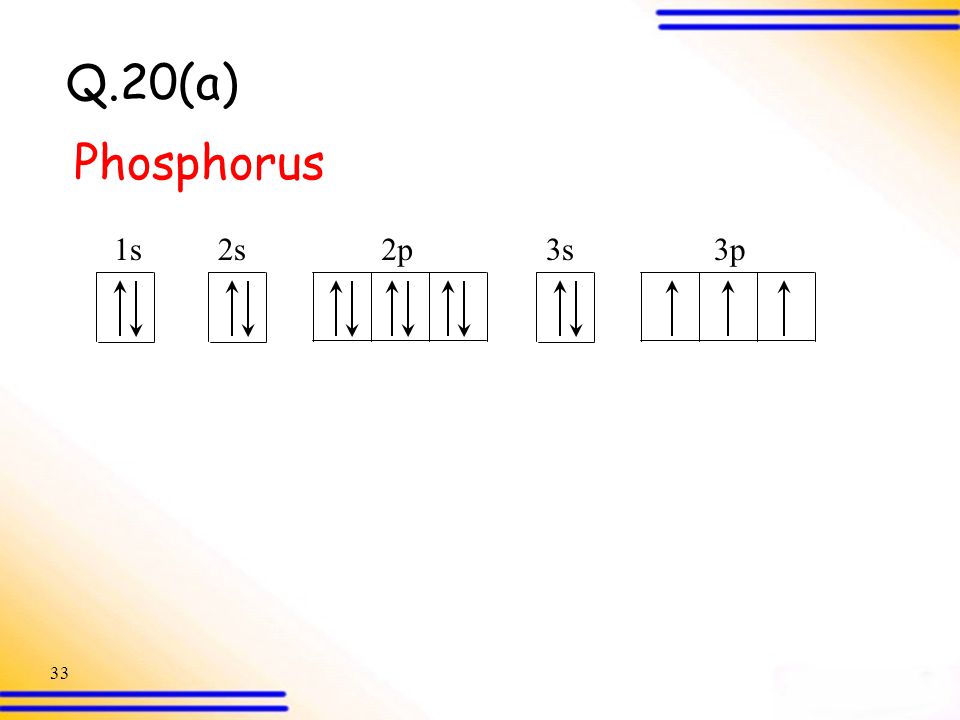
1 answerLet us start by looking at the orbital diagram of the phosphorus atom: Phosphorus atom. Phosphorus atom. As phosphorus is a non-metal, ...
Q. Enter the electron configuration for the ion most likely formed by phosphorus. ... Q. Build the orbital diagram for the ion most likely formed by phosphorus. ... Q. Write electron configurations for the most stable ion formed by each of the elements Al, Ba, Se, and I (when in stable ionic compounds). ...
0 votes. 88 views. asked Aug 25, 2020 in Other by megha00 (-1,289 points). Build the orbital diagram for the ion most likely formed by phosphorus.
Enter the electron configuration for the ion most likely formed by phosphorus. Express your answer in the order of orbital filling as a string without blank space between orbitals. For example, the electron configuration of Li could be entered as 1s^22s^1 or [He]2s^1.
This requires determining first the ground-state electronic configuration of phosphorus (P) by referring to the periodic table and locating the position of P.1 answer · Top answer: We’re asked to build the orbital diagram for the ion most likely formed by phosphorus.This requires determining first the ground-state electronic ...
Build the orbital diagram for the ion most likely formed by phosphorus? 1s22s22p63s23p3 is for Phosphorus and the most likely ion is to be a 3- because it wants to have a full outer shell ... Draw orbital diagram s for the following elements: 1.
Nov 10, 2008 — Build the orbital diagram for the ion most likely formed by ... In which orbital does an electron in a phosphorus atom experience the ...2 answers · Top answer: 15P = 1s2 2s2 2p6 3s2 3p3 15P-3 1s2 2s2 2p6 3s2 3p6









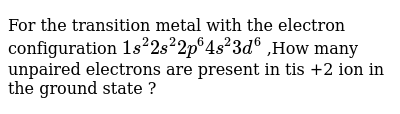







0 Response to "39 build the orbital diagram for the ion most likely formed by phosphorus."
Post a Comment