39 miscibility gap phase diagram
A detailed discussion of ternary 2-phase and 3-phase miscibility gaps was published more than half a century ago by Meijering.[4,5] The papers were published in Phillips Research Reports and contain a thorough literature review for the time.Also, they contain calculated chemical spinodal and isothermal phase diagram sections using a "Bragg-Williams thermodynamic model" (a.k.a. a regular ...
Miscibility gap. A miscibility gap is a region in a phase diagram for a mixture of components where the mixture exists as two or more phases – any region of composition of mixtures where the constituents are not completely miscible. The IUPAC Gold Book defines miscibility gap as "Area within the coexistence curve of an isobaric phase diagram ...
PHASE SEPARATION IN TRANSPARENT LIQUID-LIQUID MISCIBILITY GAP SYSTEMS by S. H. Gelles, B. N. Bhat^' and R. J. Laub^ INTRODUCTION The field of processing materials in space has provided a renewed incentive to study materials systems containing a liquid phase miscibility gap, i.e., a field in the phase diagram representing the equilibrium between two
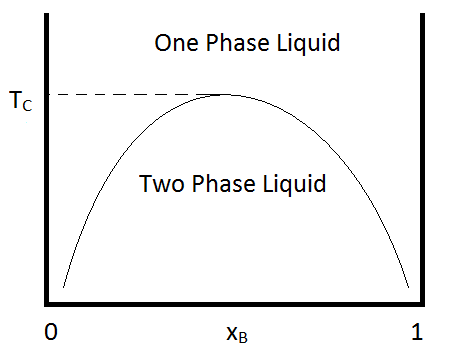
Miscibility gap phase diagram
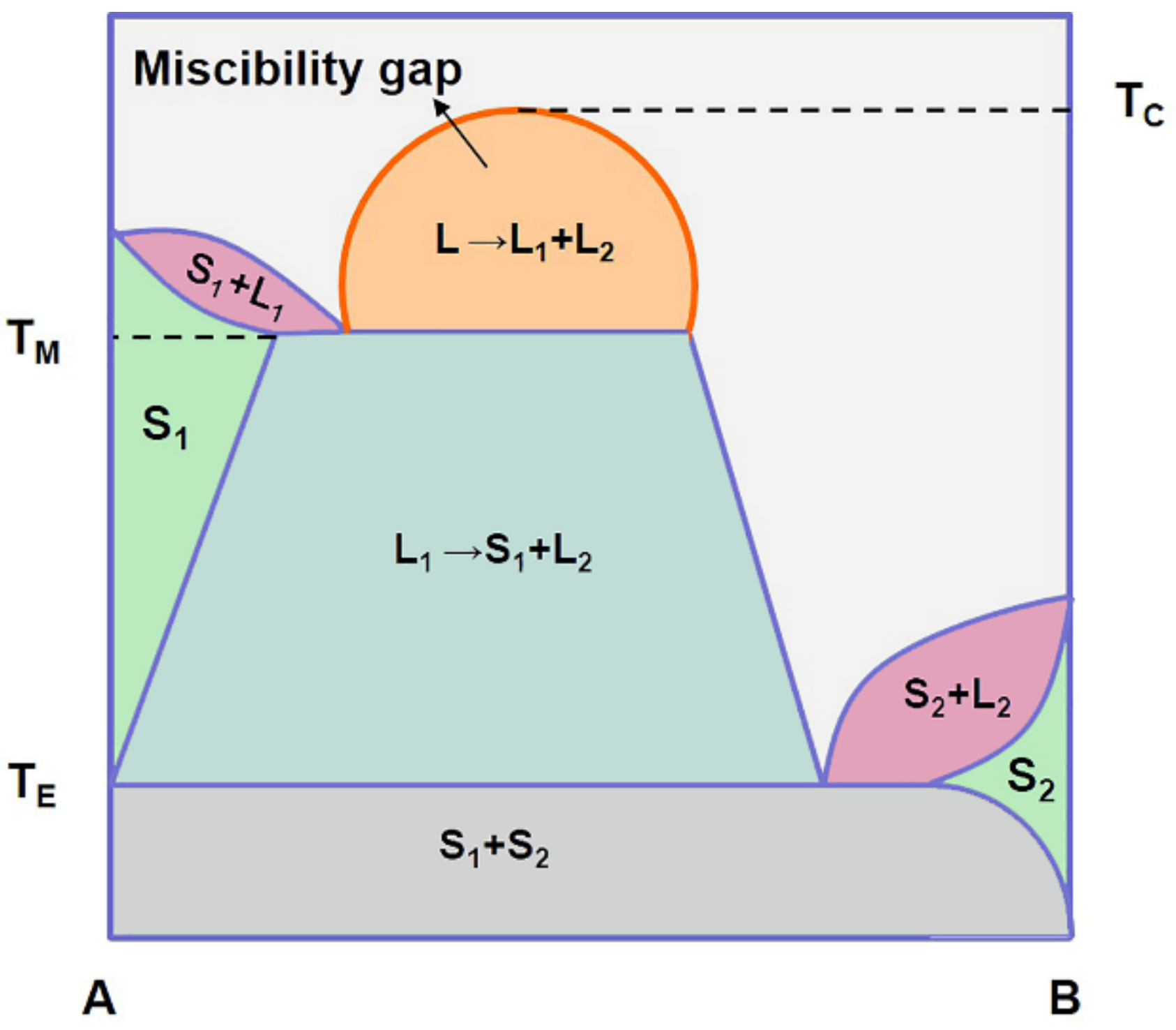
The thermodynamics of phase diagrams with a miscibility gap and a congruently melting compound and phase diagrams with two miscibility gaps are treated using the Hoch-Arpshofen solution model and the Schottky- Wagner disorder model. We extended the Schottky-Wagner disorder model to the liquid phase. In phase diagrams with two miscibility gaps, a liquid compound between the two miscibility gaps ...
for a phase described with a sublattice model in a binary system. The Al-Zn and Al-In phase diagrams are computed by using a home-made code to verify the efficiency of these techniques. The method to detect the miscibility gap in terms of interaction parameters can be generalized to sublattice models.
The region of miscibility gap is visible between 0.29 (±0.02) ≤ x ≤ 0.92 (±0.02), corresponding to an iodine rich and a bromine rich phase. Further, differentiating between a two-phase region (a) and a cluster-like region (b) to clearly describe the miscibility gap has been done and will be discussed. The pseudo cubic lattice parameters ...
Miscibility gap phase diagram.
Download scientific diagram | Copper-nickel phase diagram including the miscibility gap of the (Cu, Ni) phase: the dashed line denotes the boundary of magnetic transformation from publication ...
for low temperatures, there exists a miscibility gap separating two compositionally different solid solutions, a Na-rich and a K-rich one. Here, we have predicted the Na-rich/K-rich phase envelope in the solid part of the phase diagram using molecular simulation. The calculated phase diagram reproduces the experimental phase dia-
Some alloys whose phase diagrams do not contain a miscibility gap or a monotectic reaction form microstructures consisting of droplets embedded in a matrix of a primary phase after rapid solidification. If the liquidus curve has a portion where the slope is close to zero, a metastable miscibility gap lies just beneath the liquidus curve.
Miscibility Gap Free energy Phase diagram T 1 T 2 T 3 T 1 T 2 T 3. c F c T Miscibility Gap with liquid Free energy Phase diagram S L L. c F c T Miscibility Gap with ...
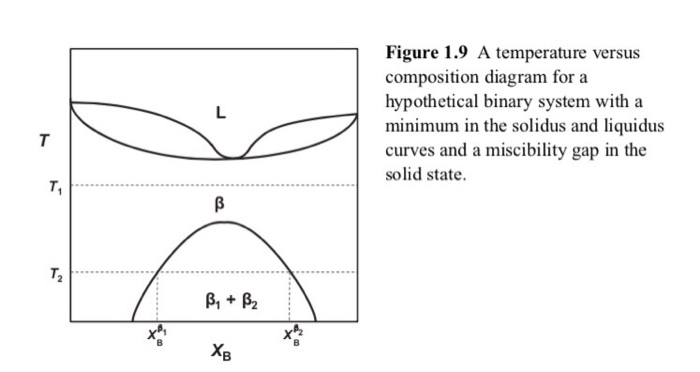
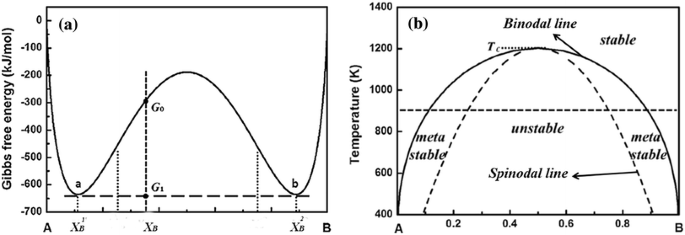
Fig. 3 Schematic binary phase diagrams with solid state miscibility where the liquidus shows a maximum (a) and a minimum (b). It also is possible to have a gap in miscibility in a single-phase field; this is shown in Fig. 4. Point Tc, above which phase α1 and α2 become indistinguishable, is a critical point. Lines a-Tc and b-Tc, called solvus ...
mutual miscibility in the solid state. Thus much of the phase diagram at low temperatures is dominated by a 2-phase field of two different solid structures- one that is highly enriched in component A (the α phase) and one that is highly enriched in component B (the β phase). These binary systems, with
This app checks the stability of liquid mixtures (binary or ternary), and calculates the mutual solubilities of the compounds in the separate liquid phases. In binary case the program plots the mutual solubilities in function of temperature, while in ternary case a ternary miscibility phase diagram is constructed (at a given temperature).
There's no miscibility gap in the phase diagram. Figure 2 Ωl=0, Ωs=1500 cal/mol. This is the case of ideal liquid and regular solid. Miscibility gap and spinodal curve are shown in the figure. Figure 3 Ωl=0,Ωs=3000 cal/mol. This is the eutectic case. Figure 4 Ωl=1500 cal/mol, Ωs=0. Figure 5 Ωl=1500 cal/mol, Ωs=1500 cal/mol.
A miscibility gap is a region in a phase diagram for a mixture of components where the mixture exists as two or more phases - any region of composition of mixtures where the constituents are not completely miscible. Thermodynamically, miscibility gaps indicate a maximum (e.g. of Gibbs energy) in the composition range.
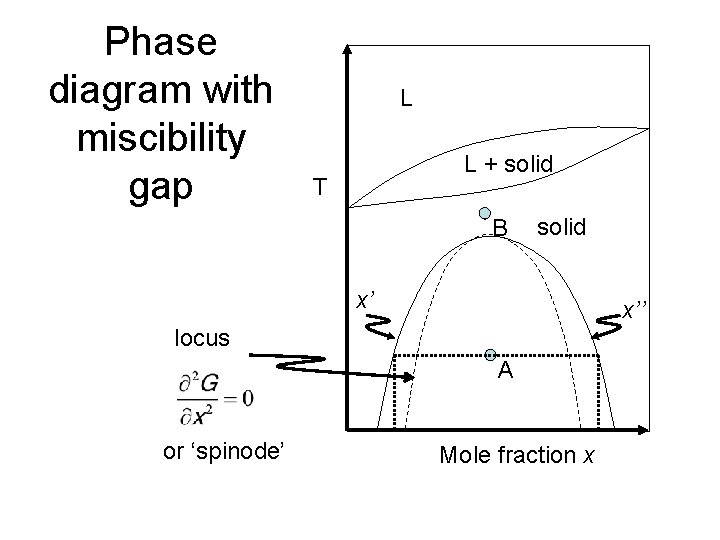
Phase diagram with miscibility gap Mole fraction x T L L + solid solid x' x'' 02 2 x G locus or 'spinode' A B 34. Example phase diagram CdTe-CdS 35.
Yes, the Cu-Ni phase diagram exhibits a miscibility gap below roughly 600 K. This has been calculated theoretically and measured experimentally: Image source. Thermodynamically, a miscibility gap is almost inevitable in metal systems, which have positive enthalpies of mixing.
Phase diagram for Gold-Nickel showing complete solid solubility above about 800oC and below about 950oC. The miscibility gap at low temperatures can be understood with a regular solution model. Figure by MIT OCW. 800 700 600 500 400 300 200 100 0 10 20 30 40 50 60 70 80 90 100 Alpha Liquid ...
Answer (1 of 2): The other answer gives a calculated phase diagram, showing a miscibility gap that develops below 300°C. Thermodynamically, such a miscibility gap is almost inevitable in metal systems, which have positive enthalpies of mixing - derivation below. In practical terms, though, it's...
A miscibility gap with x ranging from 0.4362 (4) to 0.9612 (9) is found in the Sr(Fe1−xMnx)As2 system—for x <0.4362(4), the phase remains in the parent tetragonal structure [space group I4/mmm (No. 139)], whereas for x> 0.9612(9), the phase exhibits a trigonal structure [space group P −3m1 (No. 164)].
FIU Materials Science & Engineering (MSE) graduate core courseEMA5001 Physical Properties of Materials (or Materials Kinetics & Phase Transformation)Lecture ...
Advanced Metallurgical Thermodynamics by Prof. B.S. Murty, Department of Metallurgy and Material Science, IIT Madras. For more details on NPTEL visit http:/...
[41] Fe-Cu system does not form any intermetallic compounds, and there is a wide miscibility gap between Fe and Cu in the equilibrium phase diagram. [42] As the supercooled liquid mixture...
Two Phase Regime Miscibility gap is defined by ... Temperature differentials and the "kinetic" phase diagram can lead to segregation or coring as described by Hummel. 20 Callister p. 294 Equilibrium Non-Equilibrium. 21 Mechanical Properties of Isomorphous Binary Alloy. 22
The study of phase separation in the miscibility gap and ion specific effects on the aggregation of soft matter system Li Zhang To cite this version: Li Zhang. The study of phase separation in the miscibility gap and ion specific effects on the ag-gregation of soft matter system. Soft Condensed Matter [cond-mat.soft]. Université Paris Saclay
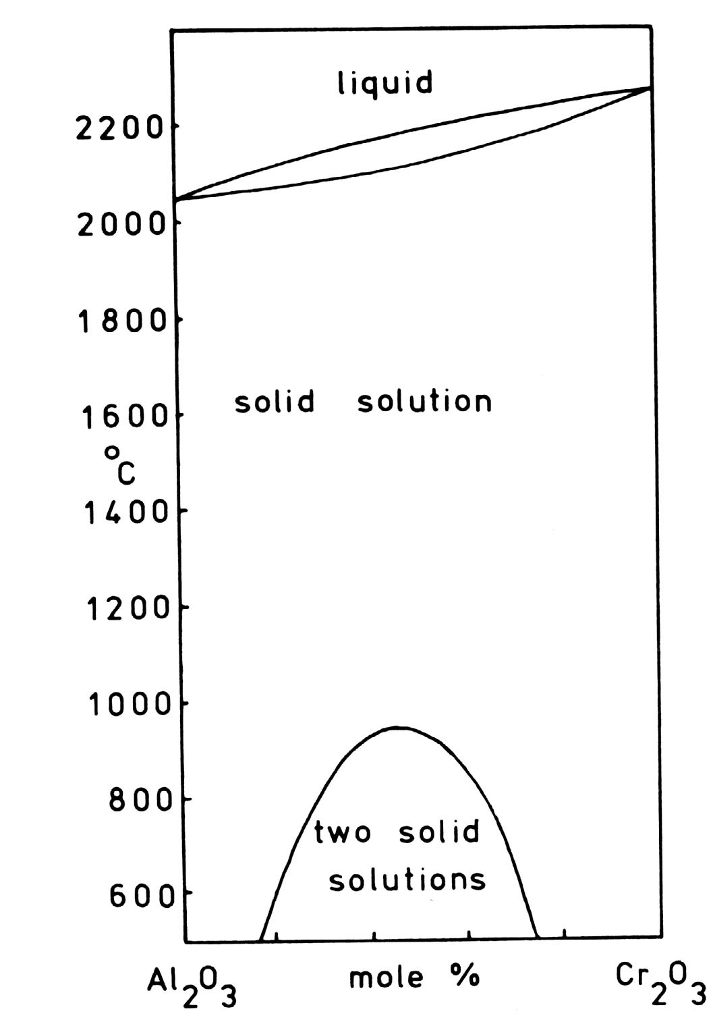
(2) A miscibility gap in the solid phase can of course occur near a pure element side (e.g., ɛ and (Cd) in the Ag-Cd phase diagram) because there may be strong crystal lattice effects. View chapter Purchase book DETERMINATION OF PHASE DIAGRAMS WITH REACTIVE OR VOLATILE ELEMENTS Cezary Guminski, in Methods for Phase Diagram Determination, 2007
We will examine diagrams with extremum points on the liquidus and solidus curves, as well as phase diagrams with a miscibility gap at low temperatures. We will learn to describe crystallization processes in the temperature interval and determine the volume fraction of phases.
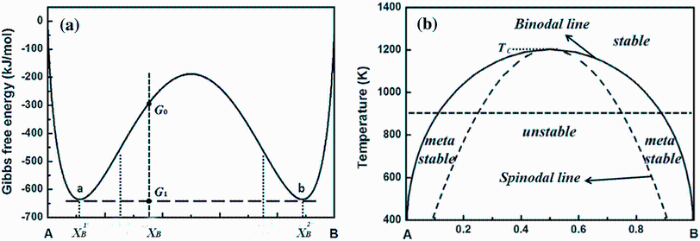
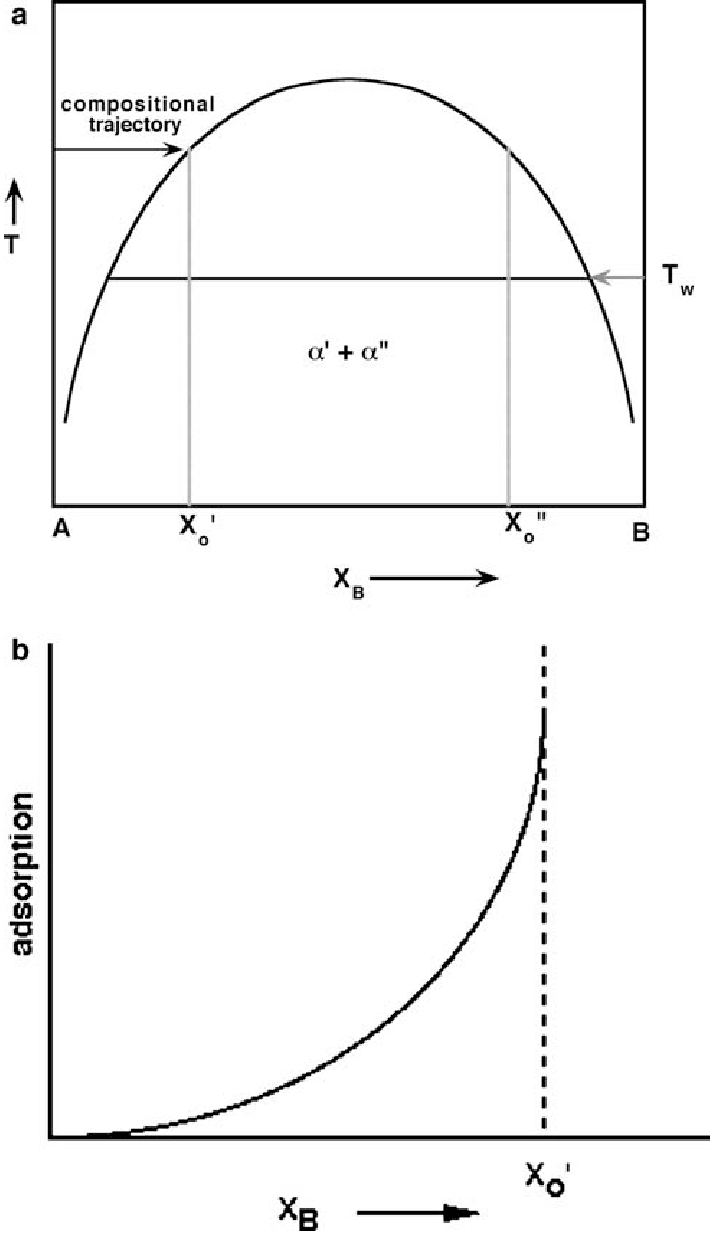
Phase Diagram and Free-energy Diagram of spinodal decomposition: As a special case of phase transformation, spinodal decomposition. can be illustrated on a phase diagram exhibiting a miscibility gap (see the diagram below). Thus, phase separation occurs whenever a material transitions into the unstable region of the phase diagram.
To some extent, rare-earth-doped UO(2) is representative of an irradiated nuclear fuel. The two phases we observed previously in neodymium-doped UO(2) are now interpreted as the existence of a miscibility gap in the U-Nd-O phase diagram using new results obtained with Raman spectroscopy. Extrapolati …


















0 Response to "39 miscibility gap phase diagram"
Post a Comment