40 electron dot diagram for br2
A step-by-step explanation of how to draw the BrCl Lewis Dot Structure.For the BrCl structure use the periodic table to find the total number of valence elec... While the Lewis Structure provides an idea about the physical attributes of the compound, its representation is limited since it is a 2-dimensional model. It also does not reflect upon the molecular design, geometry, or the 3-dimensional representation of atoms. below are the steps to draw the lewis diagram of the Br2 molecule.
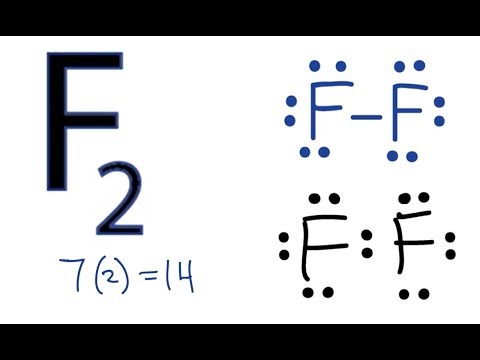
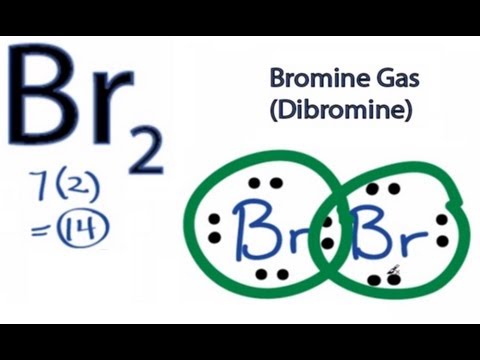
It has 7 valence electrons. We have two of them though. Multiply that by 2, for a total of 14 valence electrons for the Br2 Lewis structure. First, we'll draw two Bromine atoms next to each other. We have 14 valence electrons for Br2. We'll put two between atoms to form a chemical bond. Then we'll go around the atoms: 2, 4, 6, 8 and 10, 12, 14.

Electron dot diagram for br2
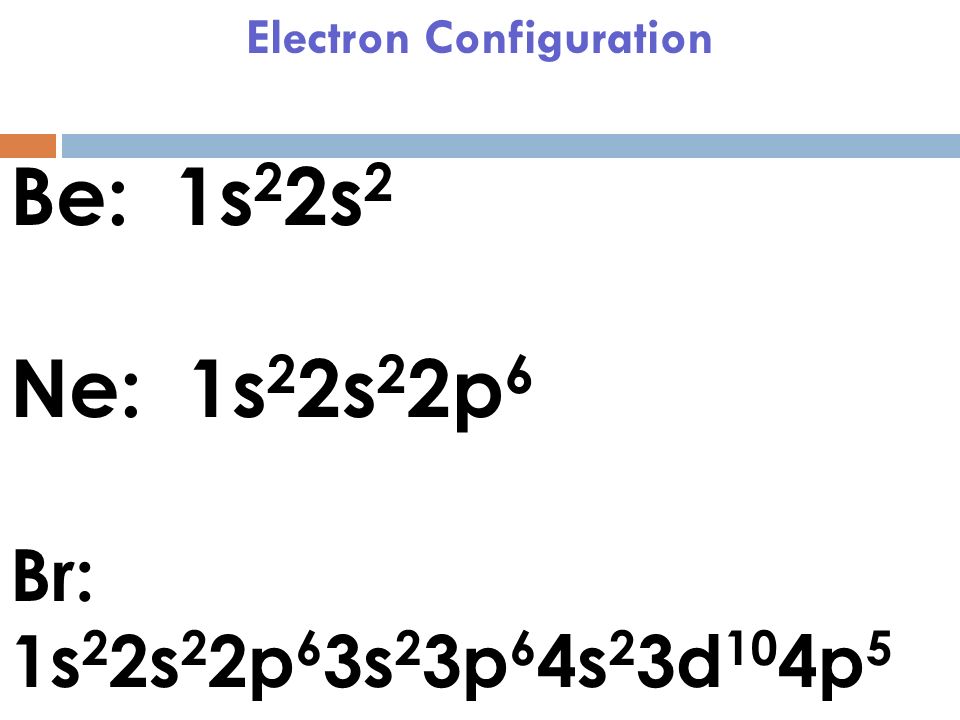
Answer (1 of 4): The easiest way to them is in steps: Step 1: Count number of total Valance electrons (12 electrons in this case) Step 2: No. of Required electrons (always 8 hence, 16 electrons) Step 3: No. of Bonding Electrons (Required electrons - valence electrons: 4 electrons in this case)... A. Draw Lewis structure for Br2 and HBr. Q1. B. What is the molecular geometr (shape of the molecule) for Br2 and HBr. Q1. C. State whether Br2 is polar or nonpolar. Q1. D. State whether HBr is polar or nonpolar. Q2. A. Draw Lewis structure for BBr Q2. B. What is the molecular geometry (shape of the molecule) for BBrs Q2. C. State whether BBr ... To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level.
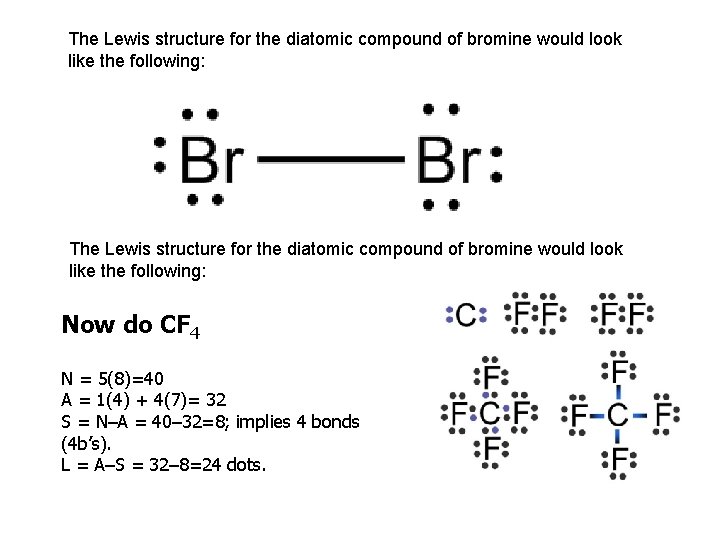
Electron dot diagram for br2. The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. Lithium fluoride, LiF 1. Lithium atom loses one electron to form the cation Li + 2. Fluorine atom gains one electron to form the anion F-3. Lithium fluoride compound can be represented as Br2 Sketch the proper Lewis structure for this substance. Be sure to follow octet/duet rules for each atom and use the total number of valence electrons available. Use your drawing to answer the following questions. Count the number of each type of electron domain on each atom. There is not a central atom here, and the two atoms are equivalent. To find the number of valence electrons in groups 13-18, you take the series number and subtract 10 from it. Therefore, if Bromine is in series 17, 17-10 is 7, so Bromine has 7 valence electrons. The electron dot diagram to the left depicts the number of valence electrons bromine has. The dots are arranged in a specific pattern to show how many ... Lewis structure of bromine contains only one Br-Br bond and each bromine atom has three lone pairs. It is very easy to Br 2 lewis structure. Br 2 lewis structure. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. So, this lewis structure is a very simple. Steps of drawing lewis structure of Br 2
A step-by-step explanation of how to draw the MgBr2 Lewis Dot Structure.For MgBr2 we have an ionic compound and we need to take that into account when we dra... Bromine | Br2 - PubChem. National Center for Biotechnology Information. 8600 Rockville Pike, Bethesda, MD, 20894 USA. Contact. Policies. FOIA. National Library of Medicine. National Institutes of Health. Department of Health and Human Services. Moving onto drawing Lewis dot structures of various compounds: Step 1: Count the total number of valence electrons in the compound. Use the periodic table to figure out how many valence electrons each individual element of the compound contributes then add them all up. Upgrade to remove ads. Draw a lewis electron-dot diagram for a molecule of br2. Draw a lewis electron-dot diagram for a molecule of br2. Answers: 2 Get Other questions on the subject: Chemistry. Chemistry, 22.06.2019 07:00, chloe9869. Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li ...
Dibromide(.1-) | Br2- | CID 5460533 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety ... Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist. The Lewis Structure for Li is Li with one dot to the right of the element. There are two types of diagrams one is the Lewis diagram the other is the Electron dot diagram. To make the electron dot diagram you put the electron symbol and put a dot on one of the sides for ...
A step-by-step explanation of how to draw the Br2 Lewis Dot Structure (Bromine gas).For the Br2 structure use the periodic table to find the total number of ...
Lewis Structure: The Lewis structure representation of an atom or a molecule is the simplest two-dimensional form of the species. It shows the element with its symbol and the electrons with dots.
Transcript: This is the Br2 Lewis structure. Steps for Writing Lewis Structures. ; In the Lewis structure for BrCl 3 there are a total of 28 valence electrons. Lewis structures depict the bonds between atoms of a molecule, as well as any unbonded electron pairs. Q1. SBr 2 is similar to the SCl 2 Lewis structure.
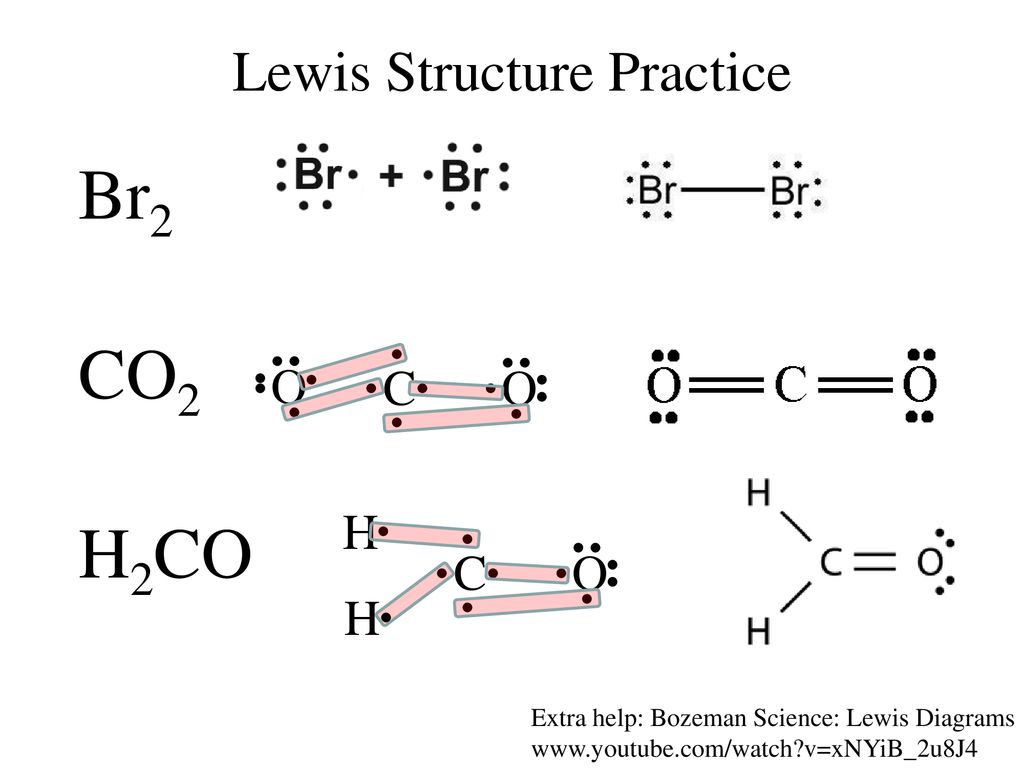
What is the correct Lewis structure for Br2?:Br - Br: What is the correct Lewis structure for O2?:O = O: What is the correct Lewis structure for N2?: N ≡ N : The Lewis structure for carbon monoxide is This structures shows. 2 lone pairs and 3 bonding pairs. Which sequence below represents the proper order of increasing bond strength?
A step-by-step explanation of how to draw the Br2 Lewis Dot Structure (Diatomic Bromine).Note that Diatomic Bromine is often called Molecular Bromine or just...
What is the electron dot structure of magnesium bromide? The structure should have single bond between Mg with the two bromides, each contributing one electron. The rest of the valence electrons are around bromide as to fulfill the octet rule of 8 electrons for each bromide attached to the magnesium.
Answer (1 of 2): First calculate the number of electrons required (ER) to give everybody an octet. That would be: ER =2*8= 16 Then calculate how many electrons you actually have. Look in the periodic table. Bromine is in column 17. Ten of that is the d block, so subtract 10. Gives 7, being the ...
Draw the Lewis structure for Br2.... Draw the Lewis structure for SiO2.... Draw the Lewis structure for OCl2.... Draw the Lewis structure for FSiN.... Other sets by this creator. Economics Unit 11 Quiz. 15 terms. webbie75. Economics Test Units 6-10. 39 terms. webbie75.
Lewis structure, electron dot diagram, electron dot structure... How do you draw the Lewis dot diagram for br2?: Br : Br : and both bromine atoms also have a pair of electrons both above and below ...
A step-by-step explanation of how to draw the I2 Lewis Dot Structure (Iodine Gas).For the I2 structure use the periodic table to find the total number of val...
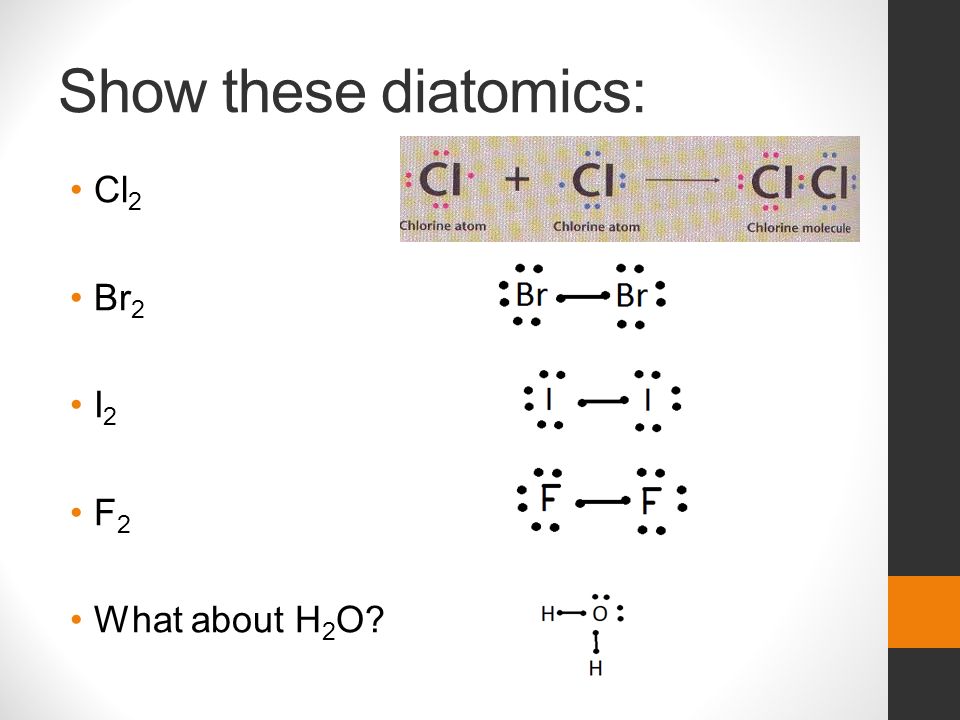
In the box below, draw the electron-dot (Lewis) structure of carbon di0>äde. In the box below, draw a Lewis electron-dot diagram for a molecule of phosphorus trichloride, PC13 (c) ammonia 60) 61) 62) 65) In the box provided, draw a Lewis electron-dot diagram for a molecule of chlorine, Ch. in terms of electrons, why the bonding in NaCl is Ionic.
To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level.
A. Draw Lewis structure for Br2 and HBr. Q1. B. What is the molecular geometr (shape of the molecule) for Br2 and HBr. Q1. C. State whether Br2 is polar or nonpolar. Q1. D. State whether HBr is polar or nonpolar. Q2. A. Draw Lewis structure for BBr Q2. B. What is the molecular geometry (shape of the molecule) for BBrs Q2. C. State whether BBr ...
Answer (1 of 4): The easiest way to them is in steps: Step 1: Count number of total Valance electrons (12 electrons in this case) Step 2: No. of Required electrons (always 8 hence, 16 electrons) Step 3: No. of Bonding Electrons (Required electrons - valence electrons: 4 electrons in this case)...



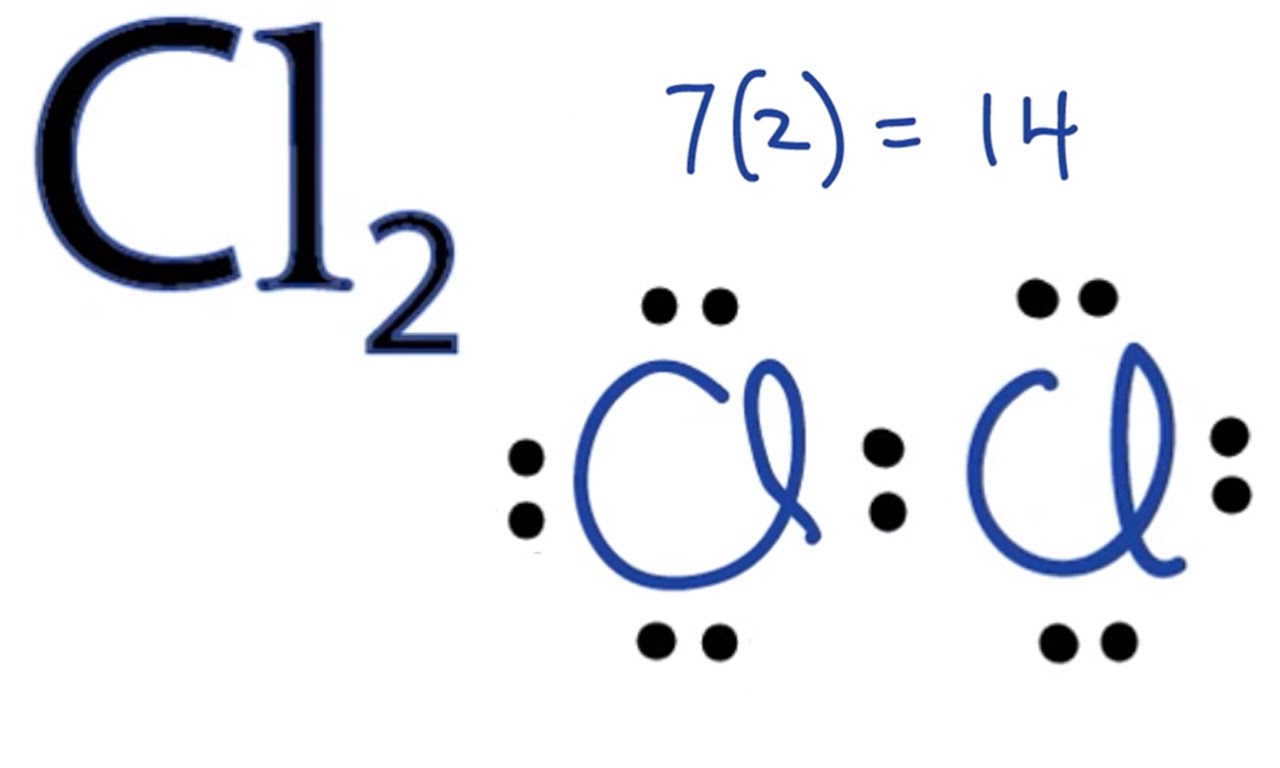


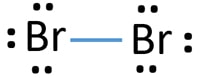
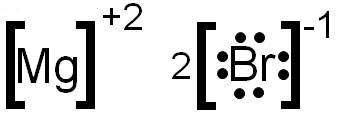



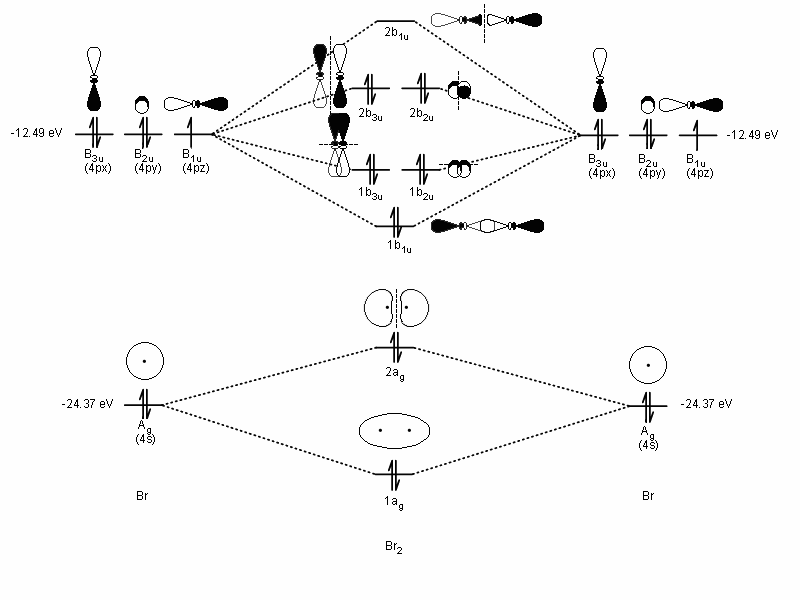











0 Response to "40 electron dot diagram for br2"
Post a Comment