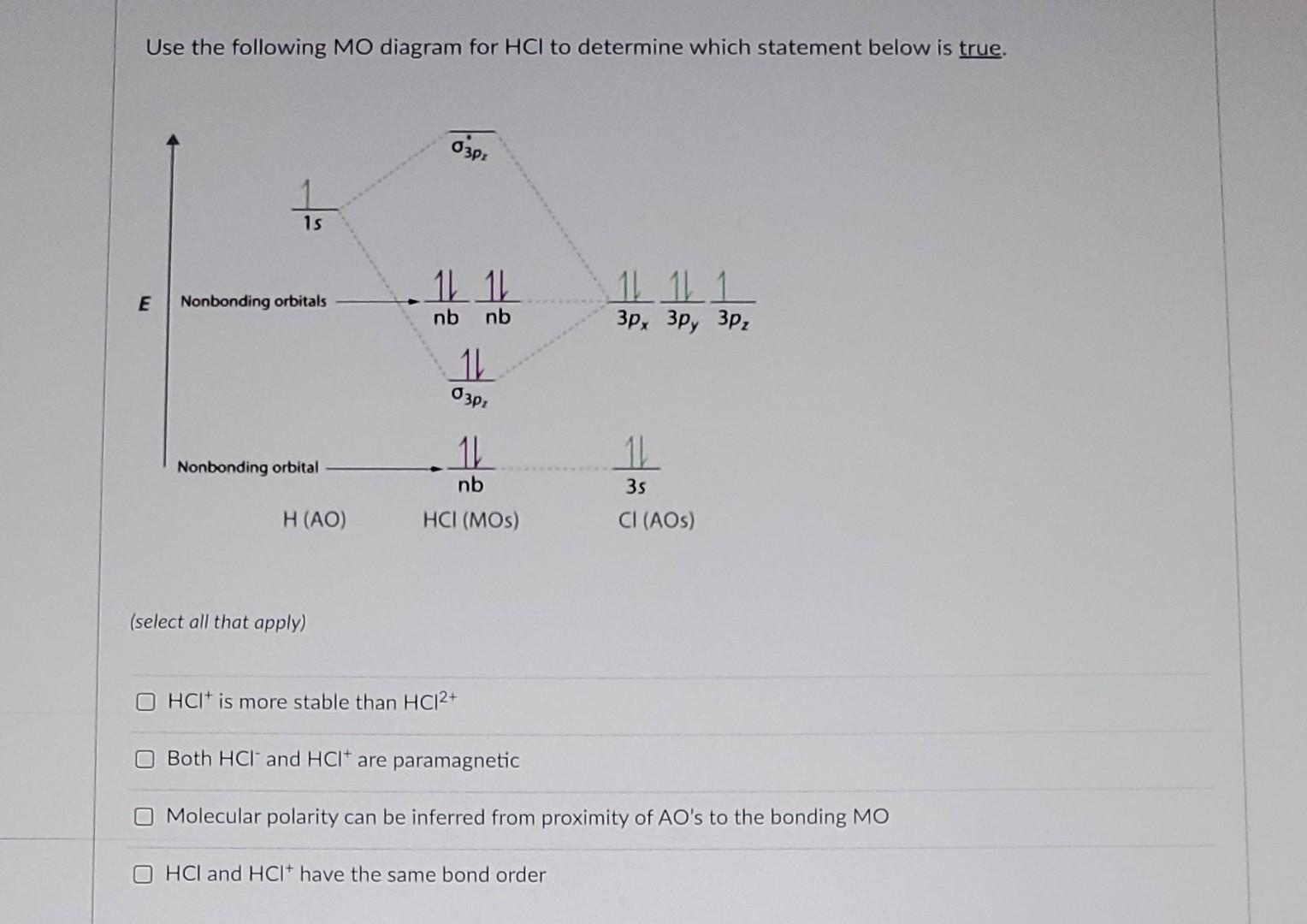
41 mo diagram for hcl
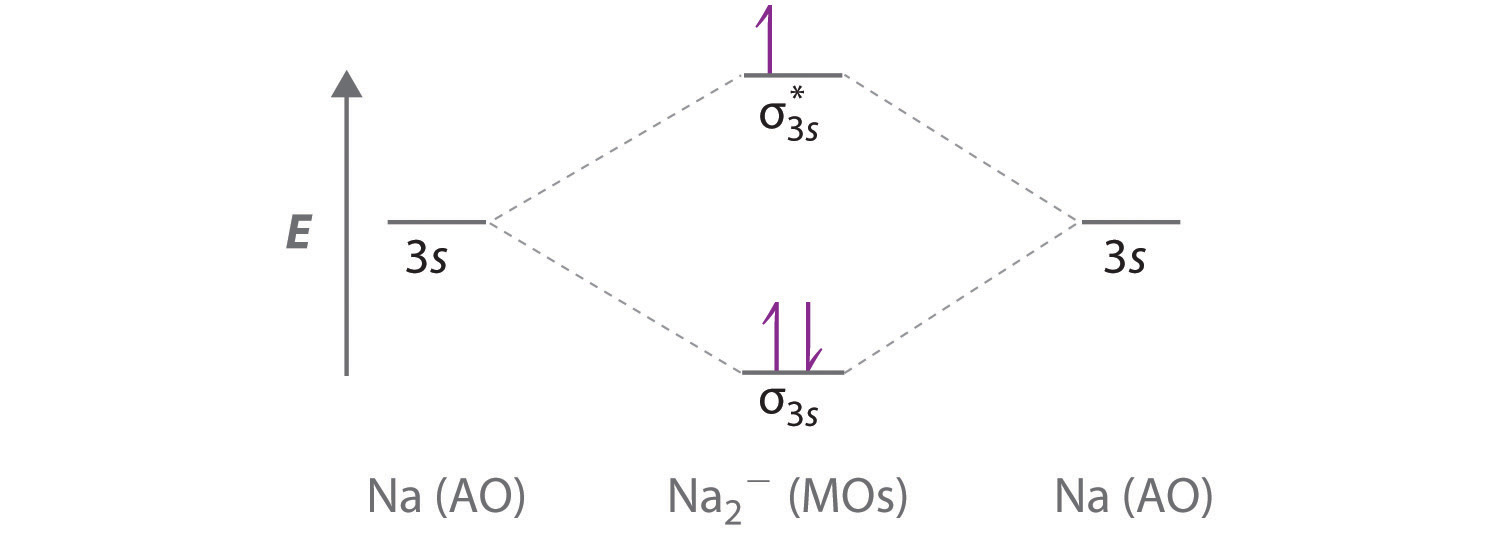
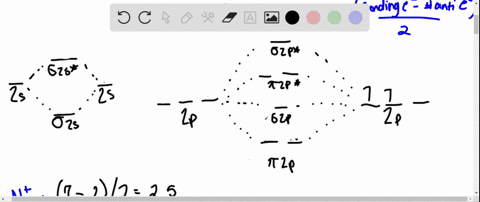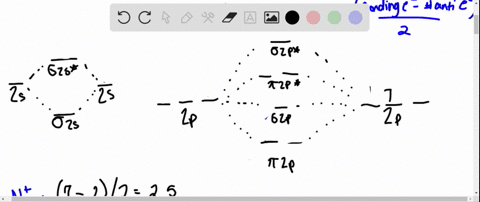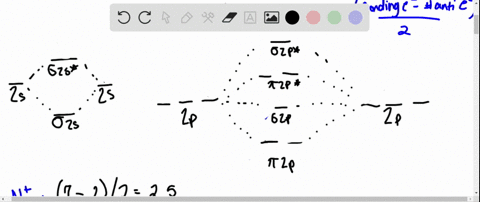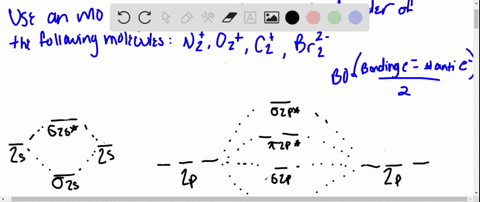
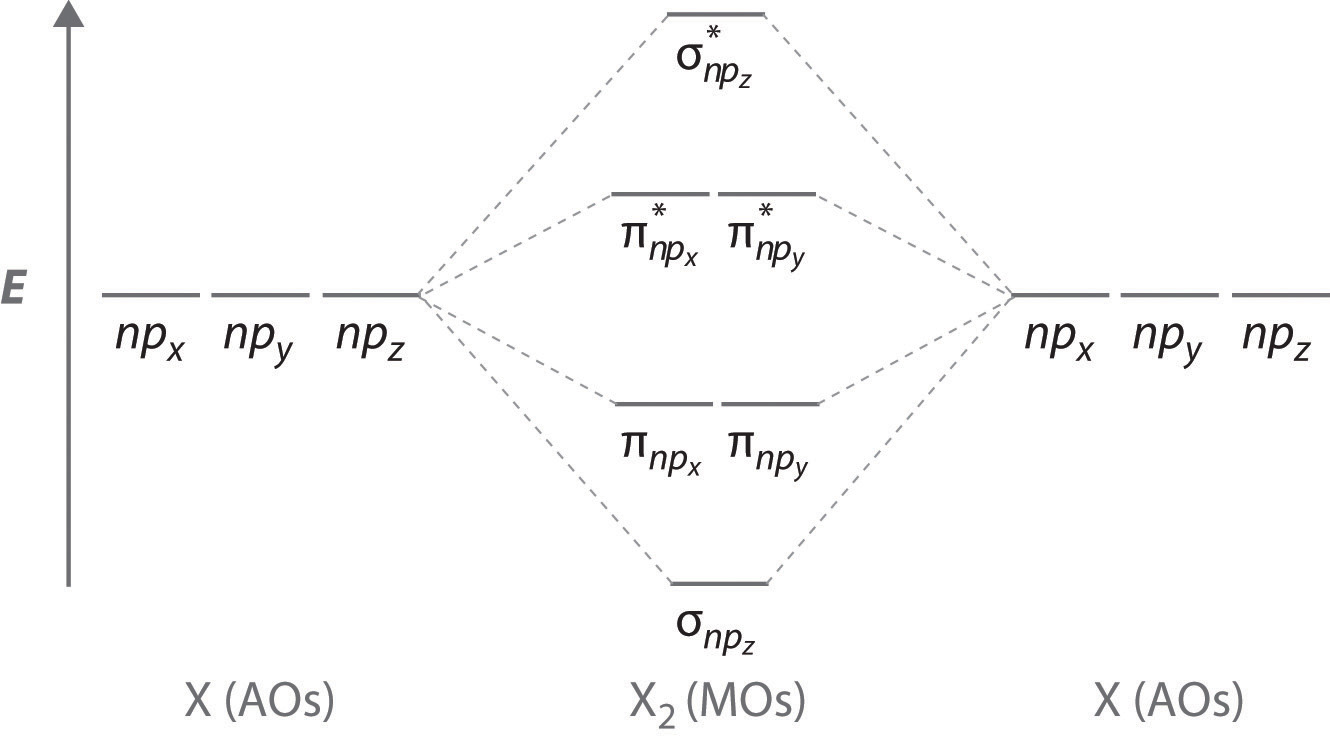
Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule. Figure 11. This shows the MO diagrams for each homonuclear diatomic molecule in the second period. The orbital energies decrease across the period as the effective nuclear charge increases and atomic ... Molecular orbital theory is also able to explain the presence of Figure \(\ PageIndex{6}\): Molecular Orbital Energy-Level Diagram for HCl. to describe the bonding in the cyanide ion (CN −). mix atomic orbitals on different atoms to get Molecular Orbitals. The resul7ng MO diagram looks like this. CN- (Cyanide ion), NO+ (Nitrosonium ion).
It is an odd rendering. I'm not sure what the actual mechanism is to create B e H X 2 but the simplistic reaction would be. B e + H X 2 ↽ − − ⇀ B e H X 2. The MO for H X 2, which is shown in the figure below is taken from Wikipedia. The right side of the diagram you showed neither represents a hydrogen molecule, nor two independent (and ...

Mo diagram for hcl
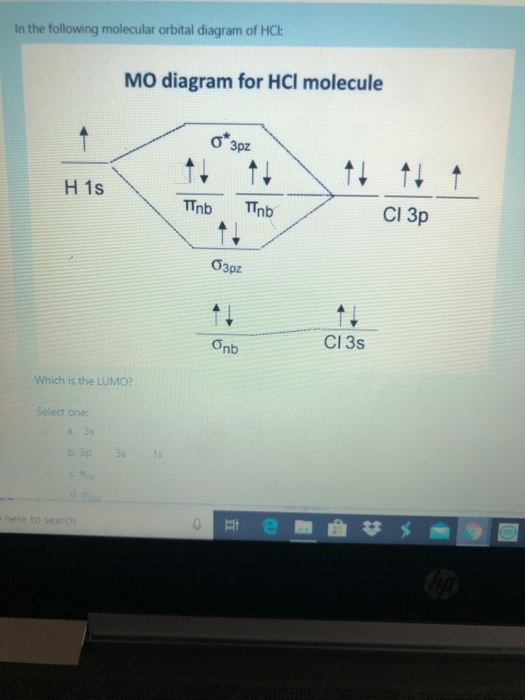
Dec 20, 2019 — Therefore, the HCL molecule has 8 pairs (1s, 2s, 2px,2py,2pz,3s,3px and 3py) of non-bonding (nb) electrons and one bonding (sigma) orbital ...2 answers · 1 vote: Answer:Cl is given to the one electrons of hydrogen and the sign is hydrogen of negative and ... Problem: Draw the MO energy diagram for HCl on your own, then use it to predict the bond order for the molecule. FREE Expert Solution. 81% (79 ratings) FREE Expert Solution. First, identify the number of valence electrons. The number of valence electron per element is based on the group number. Jan 31, · Here is a useful MO diagram of HCL found on the internet: The Cl electrons residing up to 3s orbital (1s, 2s, 2px,2py,2pz,3s) are largely stabilized than H electron in 1s orbital and therefore they cannot mix and form bond. The 3p electrons of Cl. Dec 14, · The answer is C2- because of bond orders When we draw the C2 MO, we have ...
Mo diagram for hcl. Relative AO Energies for MO Diagrams H He Li Be B C N O F Ne B C N O F Ne Na Mg Al Si P S Cl Ar Al Si P S Cl Ar 1s 2s 2p 3s 3p -19.4 eV -15.8 eV -32.4 eV -10.7 eV Photoelectron spectroscopy gives us a pretty good idea of the relative energies for AOs. Chlorine is sp3-hybridized, and so there are four tetrahedrally-arranged 3sp3 orbitals surrounding it.Hydrogen does not hybridize, so it's simply a circle fo... MO Diagram of HCl : In case of MO diagram of HCl, combination between the hydrogen 1s AO and chlorine 1s, 2s,2p and 3s orbital can be ruled out because their energies are too low. If the overlap occur between the chlorine 3p y and 3p z orbitals it would be non bonding because the positive lobe of hydrogen will overlap equally with positive and ... Figure 11.5. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Part (a) in Figure 11.5. 3 shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
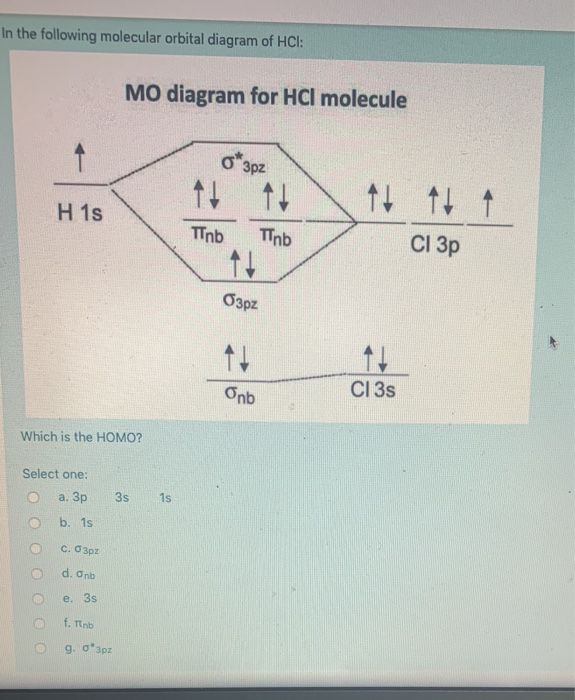
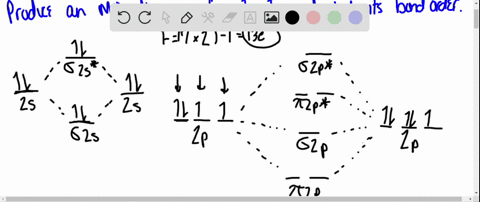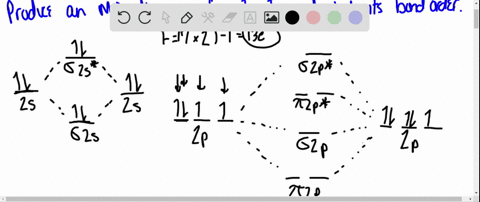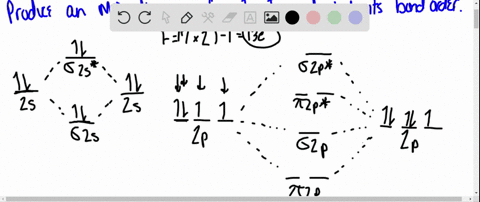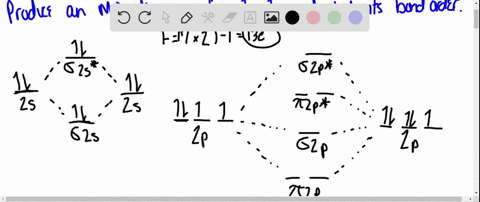
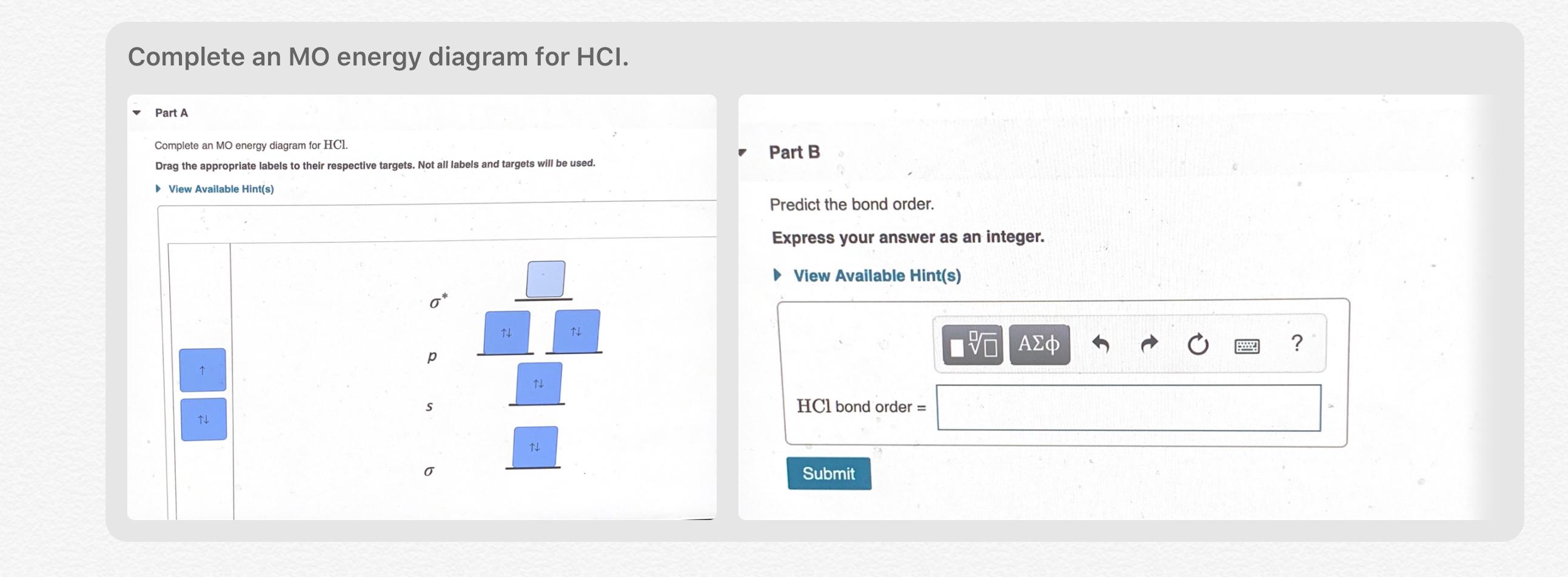
MO DIAGRAM OF HCl For understanding plz visit my YouTube videos click here -- https://youtu.be/pNLeI4TRK98 Fe-II was stable in 1 M HCl at room temperature for several days, whereas in 6 M HCl at 70 degrees C, 90% of the Fe-II was oxidised within 24 h. In the absence of O-2, no Fe-II oxidation occurred. Problem 54 Hard Difficulty. Draw an MO energy diagram for HCl. Predict the bond order and make a sketch of the lowest energy bonding molecular orbital. The 𝐻𝐻−𝐶𝐶 bond order𝑒𝑒 calculated from the MO diagram is . 2−0 2 = 2 2 = 1. The Lewis diagram shows three pairs of nonbonding electrons on Cl. The MO diagram shows three pairs of nonbonding electrons in MOs localized on Cl. The two bonding theories therefore give the same picture of 𝐻𝐻. 𝐶𝐶𝑒𝑒. H Cl H HCl ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... "HCl" has no orbital hybridization. Chlorine's 3s is too low in energy to interact with hydrogen's 1s, but chlorine's 3p_z can interact with hydrogen's 1s atomic orbital just fine. A good general rule is that being less than about 12 eV apart in energy is required for orbitals to be close enough in energy. The 3s and 3p orbitals of "Cl" are apparently too far apart in energy to interact for ... A simple approach to molecular orbital (MO) theory for heterogeneous diatomic molecules is to show the energy level diagram. The MO energy levels can be worked out following these steps: Recall that the energy E n for the quantum number n is for an element with atomic Z is approximately. (1) E n = 13.6 Z E f f 2 n 2 e V. Answer (1 of 2): Here is a useful MO diagram of HCL found on the internet: The Cl electrons residing up to 3s orbital (1s, 2s, 2px,2py,2pz,3s) are largely stabilized than H electron in 1s orbital and therefore they cannot mix and form bond. The 3p electrons of Cl have comparable energy with the ...
Module Two Chem 101 Problems. Carbon dioxide is a _____ compound composed two types of _____ atoms. Carbon dioxide is a molecular compound composed of two oxygen atoms with covalent double bonds to a central carbon atom. Both C and O are to the right of the "staircase" on the periodic table, as nonmetals. Classify the following compounds as ...
MO Diagram of CCl4. A MO diagram is nothing but a representation of bonds that are formed within the atoms to form a compound. This diagram is based on Molecular orbital theory. With the help of a MO diagram, the existence of certain compounds can be explained. Here is the pictorial representation of how CCl4's and CH4 MO diagram looks like.
Molecular Orbital Theory and MO diagram of Dibromine (Br2) The MO diagram or Molecular Orbital diagram is an extension of the 3-dimensional molecular design and gives a better understanding of the structure of an atom. Molecular Diagram also reflects upon bond length, bond shape, bond energy, and the bond angle between 2 atoms.
Molecular Orbital Theory: Heteronuclear Diatomic Molecules . 1. Energy Values for the Atomic Orbitals of Hydrogen and Helium Atoms . Hydrogen Helium 1s -1.00 Ry -1.81 Ry (a) Use the energy values in the table above to help you develop a valencemolecular orbital energy level diagram for the helium protonate ion (𝐻𝐻𝐻𝐻𝐻𝐻+).
How are the 3p orbitals of chlorine lower in energy than the 1s orbital of hydrogen? MO diagram of HCl. Share. Share a link to this question. Copy link1 answer · Top answer: I'd say there are two important things to consider here: Firstly, Cl is more electronegative than H. And secondly, as you proceed from the left to the ...
14. Compare the three bond orders (Lewis dot structure, MO diagram, computer model calculated). Explain any differences. Comparison of O 2 and NO computational models: 15. Explain why there is a difference in bond length between O 2 and NO. Use your molecular orbital diagrams and the results of the computations as a basis for your answer.
energy level diagram is similar to that of NO (Problem 5.7) without the antibonding π* electron. b. The bond order is three, with no unpaired electrons.29 pages
Molecular Orbital Diagram - Cl2, Br2, I2 3s & 3p and higher atomic orbitals are not so widely separated in energy and allow significant mixing (hybridization) to occur. This mixing causes the inversion of the σσand πmolecular orbitals' energy. σσσ ππ σ* π* 3,4,5 p 3, 4,5 s σ* σ 3,4,5 s 3,4,5 p Interhalogens Br Br F F Br F F F F.
Valence Bond Model vs. Molecular Orbital Theory . Because arguments based on atomic orbitals focus on the bonds formed between valence electrons on an atom, they are often said to involve a valence-bond theory.. The valence-bond model can't adequately explain the fact that some molecules contains two equivalent bonds with a bond order between that of a single bond and a double bond.
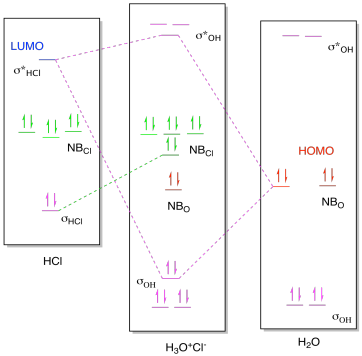
Because these orbitals have the same symmetry (in the point group of the molecule), they can make the bonding and antibonding MO combinations; The MO Diagram of \(HCl\) Note that there is 1 bond and three pairs of nonbonding electrons; The nonbonding orbitals are localized on the \(Cl\) atom
This lecture clearly explains the molecular orbital diagram of heteronuclear diatomic molecule (HF & HCl). This will help students of H.S., BS-MS, B. Sc., M....
Aug 20, 2015 · 2 answersHere is a useful MO diagram of HCL found on the internet: The Cl electrons residing up to 3s orbital (1s, 2s, 2px,2py,2pz,3s) are largely stabilized than H ...What is the molecular orbital diagram of HCl and ...1 answerDec 11, 2017What is the electronic configuration of an HCL ...1 answerDec 24, 2017Which is the molecular orbital diagram for HF? - Quora3 answersOct 21, 2016How do we draw the molecular orbital diagram of ...1 answerMay 3, 2020More results from www.quora.com
Molecular Orbital Diagram Wikipedia The Free Encyclopedia Diagram Molecular Science Chemistry . How Do You Know If Hbr And Hf Is Within 13 Ev In Chegg Com . What Is The Molecular Orbital Diagram For Hcl Quora . Answered What Is The Expexted Major Product From Bartleby . What Is The Molecular Orbital Diagram For Hcl Quora
Jan 31, · Here is a useful MO diagram of HCL found on the internet: The Cl electrons residing up to 3s orbital (1s, 2s, 2px,2py,2pz,3s) are largely stabilized than H electron in 1s orbital and therefore they cannot mix and form bond. The 3p electrons of Cl. Dec 14, · The answer is C2- because of bond orders When we draw the C2 MO, we have ...
Problem: Draw the MO energy diagram for HCl on your own, then use it to predict the bond order for the molecule. FREE Expert Solution. 81% (79 ratings) FREE Expert Solution. First, identify the number of valence electrons. The number of valence electron per element is based on the group number.
Dec 20, 2019 — Therefore, the HCL molecule has 8 pairs (1s, 2s, 2px,2py,2pz,3s,3px and 3py) of non-bonding (nb) electrons and one bonding (sigma) orbital ...2 answers · 1 vote: Answer:Cl is given to the one electrons of hydrogen and the sign is hydrogen of negative and ...






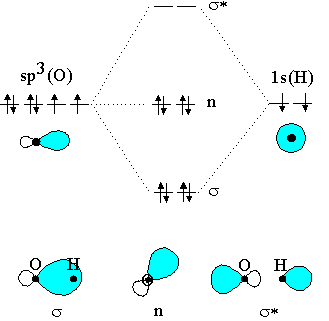



















0 Response to "41 mo diagram for hcl"
Post a Comment