42 lewis dot diagram calculator
Pictorial representations are often used to visualize electrons, as well as any bonding that may occur between atoms in a molecule. In particular, chemists use Lewis structures (also known as Lewis dot diagrams, electron dot diagrams, or electron structures) to represent covalent compounds.
Search on topics your interested. Read great personalised news.
• (3c) ·Lewis dot diagrams are used to represent valence electrons in an element. Structural formulas show the arrangements of atoms and bonds in a molecule and are represented by Lewis dot structures. • Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding.

Lewis dot diagram calculator
November 20, 2020 - Is there some sort of calculator that generates lewis structures based on valence electrons, and not the formula name itself?
Demos > Lewis Dot Structures. This demo will convert a skeletal figure, provided by a drawing in the HTML5 SketcherCanvas component on the left, into a Lewis Dot Structure in the Canvas on the right. When you are finished drawing your 2D structure, click on the Get Lewis Dot Structure button to see the result.
Lewis Dot Structures. This demo will convert a skeletal figure, provided by a drawing in the HTML5 SketcherCanvas component on the left, into a Lewis Dot Structure in the Canvas on the right. When...
Lewis dot diagram calculator.
Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
The Lewis Electron Dot Structures Concept Builder is shown in the iFrame below. There is a small hot spot in the top-left corner. Clicking/tapping the hot spot opens the Concept Builder in full-screen mode. Use the Escape key on a keyboard (or comparable method) to exit from full-screen mode. There is a second hot-spot in the lower-right corner ...
However, before going through the Lewis dot structure generator, you need to know about it in depth. Beginning with a diagram that represents only molecule associations (single bonds), you can work on building a Lewis dot structure by utilizing this generator.
Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1.
January 23, 2021 - It's a very simple drawing. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule.
Draw a Lewis Dot Diagram that would work for the elements in the Nobel Gas family (use a circle as the center) True or false even though Helium is in the Noble Gas family it has 2 valence electrons True (there are only 2 ve in the first ring). Argon is a noble gas with the atomic symbol Ar, atomic number 18, and atomic weight It is used in ...
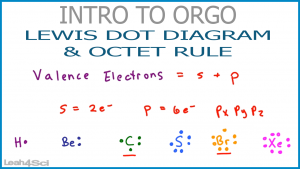
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Electrons exist outside of an atom's nucleus and are found in principal energy levels that contain only up to a specific number of electrons.
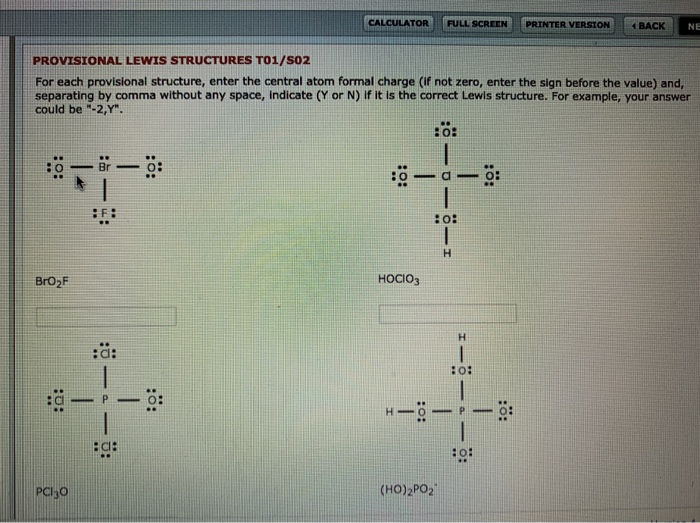
the stmcture than what is calculated in step 1. A 3- charge means that the Lewis dot structure will have 3 MORE electrons than what is calculate in step 1. Once the structure is completed, a bracket [ will be placed around the entire structure. 4. 5. 6. Complete the Lewis dot structure for 1-130+ Complete the Lewis dot structure for OH-
Welcome to the Molecular Structure Calculations Database. There are about 1000 calculations in the database, currently. Use this page to find the results that interest you. Fill in the form below with as much information as you like. Leave the information blank if you want to find several possible formulas.
‹ Lewis Dot Structures up Resonance Structures › · These tutorials are sponsored by PhySy, the maker of PhySyCalc on iPhone, iPad, or Mac OS, and RMN on Mac OS. PhySyCalc is the only calculator app that let's you use units directly in calculations. RMN is an intuitive multi-dimensional ...
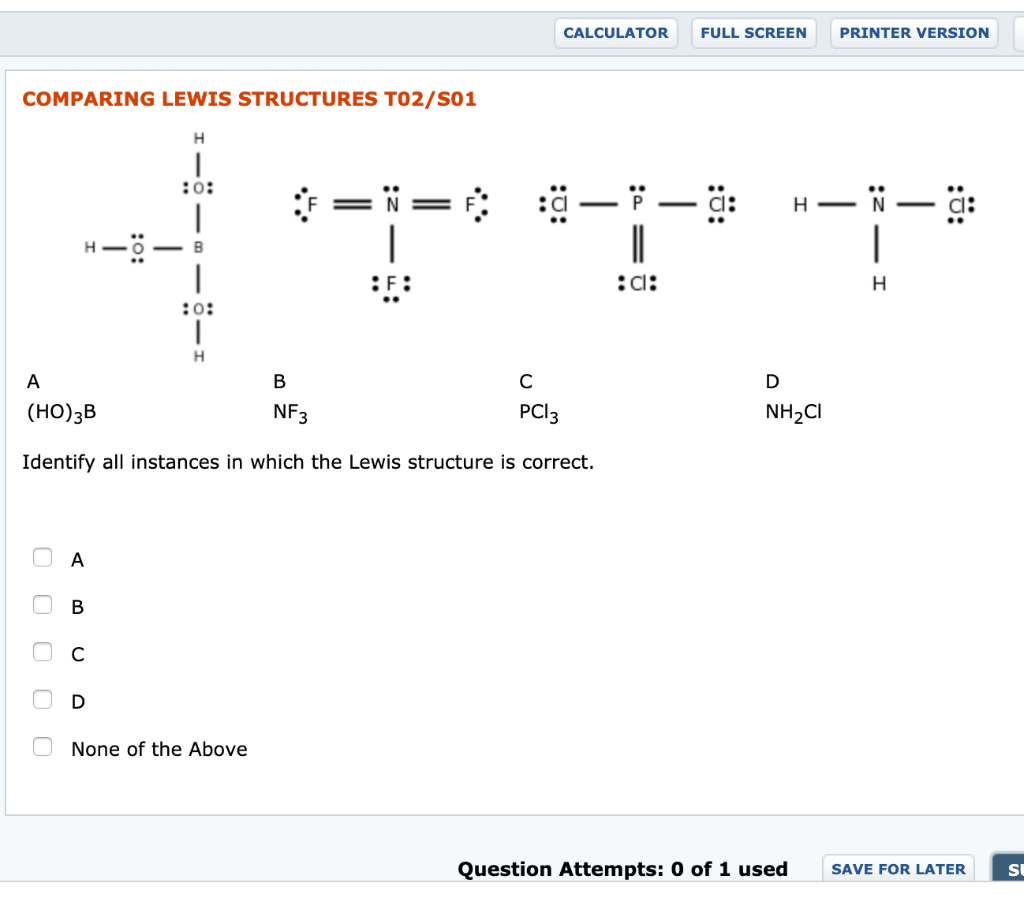
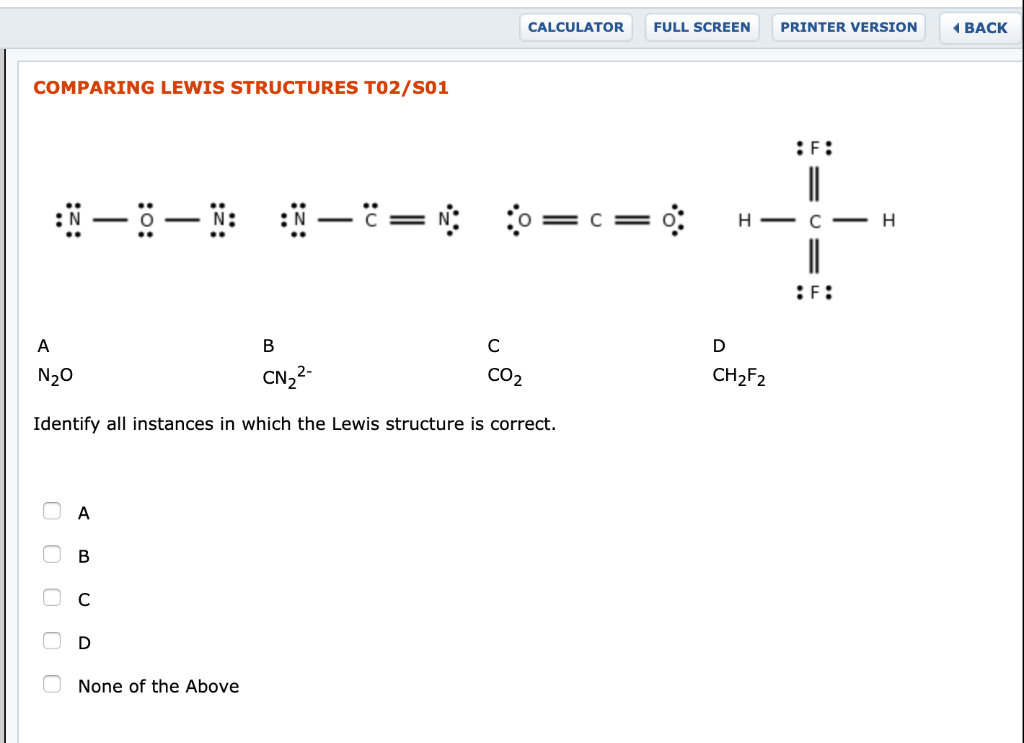
Lewis Structures, Shapes, and Polarity W 319 Everett Community College Student Support Services Program Draw Lewis structures, name shapes and indicate polar or non-polar for the following molecules: a. CH 4 b. NCl 3 c. CCl 2 F 2 d. CF 2 H 2 e. CH 2 O f. CHN g. PI 3 h. N 2 O i. SO 2 j. CS 2 k. CO l. H 2 O m. COF 2 n. N 2 o. O 2 p. H 2 q. Cl 2 r ...
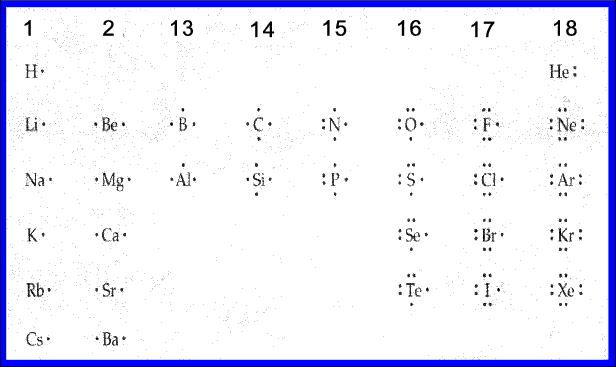
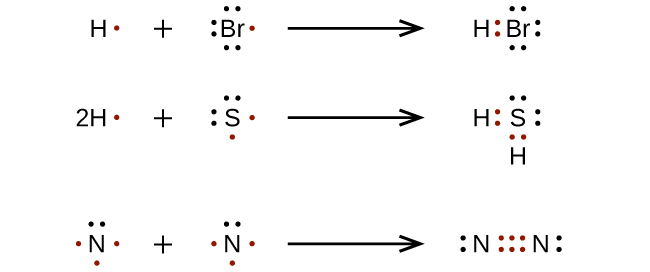
Lewis dot diagrams for elements are a handy way of picturing valence electrons, and especially, what electrons are available to be shared in covalent bonds. The valence electrons are written as dots surrounding the symbol for the element: one dot is place on each side first, and when all four positions are filled, the remaining dots are paired ...
Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also . Calculate the total number of valance shell electrons (n1) in all constituent atoms or ions. For example, in NHX4X+, n1 will be 8 (5 of N, 4 from all H's . Martin Lewis has warned pension savers
April 27, 2017 - If you do not already know the name or chemical formula of the molecule you wish to generate a dot structure for, you can browse the list of molecules at molcalc.org by clicking here. Once you are ready to generate the diagram, head over to our JavaScript API, which makes use of WolframAlpha ...
It is sometimes useful to calculate the formal charge on each atom in a Lewis structure. The first step in this calculation involves dividing the electrons in each covalent bond between the atoms that form the bond. The number of valence electrons formally assigned to each atom is then compared ...
August 1, 2018 - Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
Lewis Structures of Polyatomic Ions Building the Lewis Structure for a polyatomic ion can be done in the same way as with other simple molecules, but we have to consider that we will need to adjust the total number of electrons for the charge on the polyatomic ion.
• The Lewis dot structure famously predicts the wrong electronic structure for O2 • We can use LCAO-MO theory to get a better picture: 2s a 2p a 2s b 2p b Notice that Eσ> Eπ, because the σ bonds have more overlap than π bonds Eσ Eπ
A step-by-step explanation of how to draw the NH2CH2CHO Lewis Dot Structure.For the NH2CH2CHO structure use the periodic table to find the total number of va...
However, Lewis dot structures and hybridization are approximations that may or may not match reality. We should verify the usefulness of our simple predictions with molecular orbital theory. If the theoretical calculations are done carefully, we can learn a lot about chemical structure by comparing our Lewis structures and hybridization ...
This chemistry video tutorial provides a basic introduction into drawing lewis dot structures but most importantly, it provides an explanation on how to calc...
October 6, 2020 - Use this accurate and free Lewis Dot Structure Calculator to calculate any problems and find any information you may need.
A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures. The periodic table has all of the information needed to draw a Lewis dot structure. Each Group, or column, is indicated by a roman numeral which represents the number of valence electrons.
A Lewis dot structure is a drawing of a molecule. The drawing only "works" f0r stable molecules that actually exist. So it's a nice tool to explore how atoms bond into more complex substances. A Lewis dot structure is also called a Lewis structure, a Lewis dot diagram, an electron dot structure, or a dot diagram.
Lewis dot diagrams for elements are a handy way of picturing valence electrons, and especially, what electrons are available to be shared in covalent bonds. The valence electrons are written as dots surrounding the symbol for the element: one dot is place on each side first, and when all four ...
Electron Distributions Into Shells for the First Three Periods. A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral.
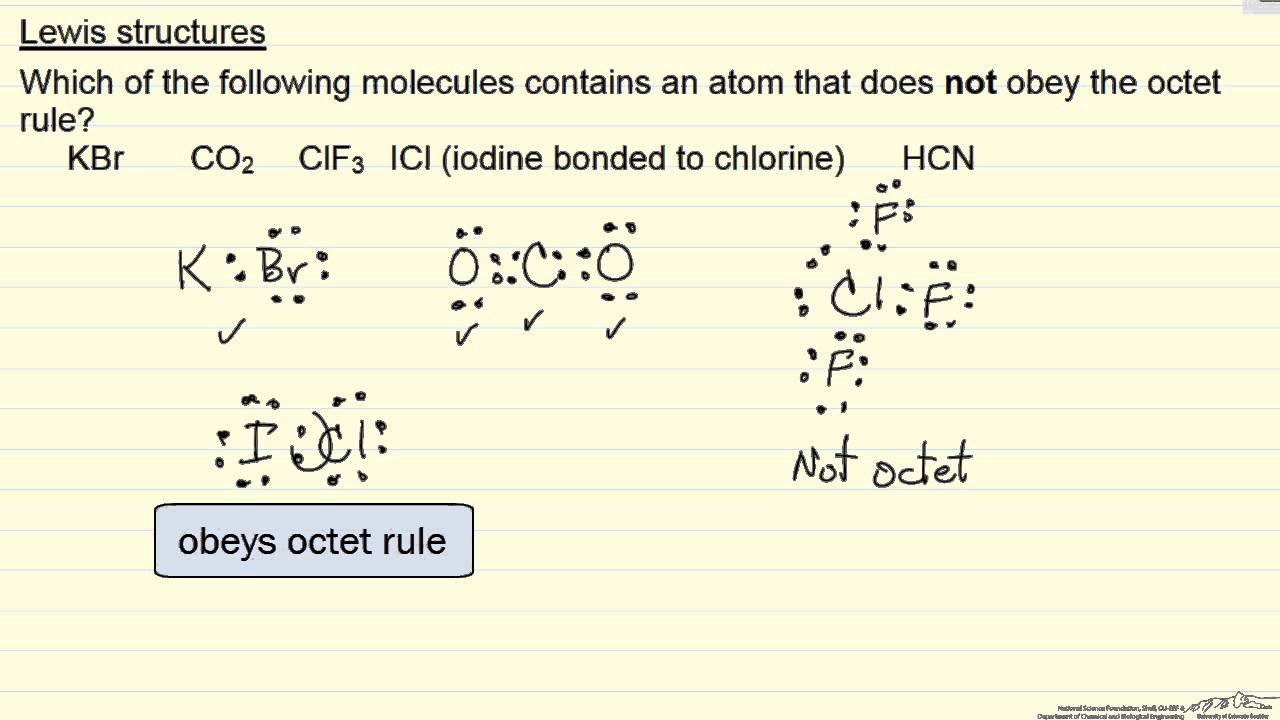
Lewis diagrams. Drawing Lewis diagrams. Worked example: Lewis diagram of formaldehyde (CH₂O) Worked example: Lewis diagram of the cyanide ion (CN⁻) Exceptions to the octet rule. Worked example: Lewis diagram of xenon difluoride (XeF₂) Practice: Lewis diagrams. This is the currently selected item. Next lesson.
Lewis Dot Structure Calculator - fasrplace ... fasrplace
Ketzbook demonstrates how to draw Lewis diagrams for elements and simple molecules using an easy to follow step-by-step explanation with several examples. Le...
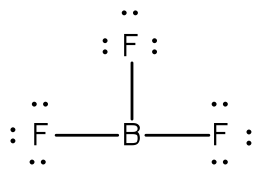
Lewis Dot Structure (Electron Dot Structure) A Lewis dot structure is a quick and easy diagram that shows the valence electrons in an element. In a Lewis structure, the nucleus of the element is represented by its symbol. The valence electrons are represented by dots placed around the symbol in pairs.
Pictorial representations are often used to visualize electrons, as well as any bonding that may occur between atoms in a molecule. In particular, chemists use Lewis structures (also known as Lewis dot diagrams, electron dot diagrams, or electron structures) to represent covalent compounds.
October 31, 2021 - Enter the total number of valence electrons, lone pairs of electrons, and total number of bound electrons to calculate the formal charge.
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
Draw the four bonds in the structure. O C O O C O Lewis Dot Structure of ClO4- by Bonds Table B. Number of Bonds. # bonds = (40- 32) = 8 = 4 bonds 2 2 C. Remaining electrons. Remaining e- = 32 - 8 = 24 e-Remaining Writing Lewis Structure: 6. Place the remaining 24 electrons in the structure such that each atom has an octet to complete the Lewis ...
Lewis Dot Structures can be produced by following a sequence of steps. Let's produce a Lewis Dot Structure for: NH 4 + (the ammonium ion). Step 1: Count valence electrons: N = 5 4 x H = 4 x 1 = 4 "+" = -1 Total = 5+4-1= 8 electrons = 4 bonds and lone pairs. Step 2:!Arrange the atoms (identify a central atom, if possible).
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Steps to use Lewis Structure Generator:-. Follow the below steps to get output of Lewis Structure Generator. Step 1: In the input field, enter the required values or functions. Step 2: For output, press the “Submit or Solve” button. Step 3: That’s it Now your window will display the Final Output of your Input. More Online Free Calculator.



/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)





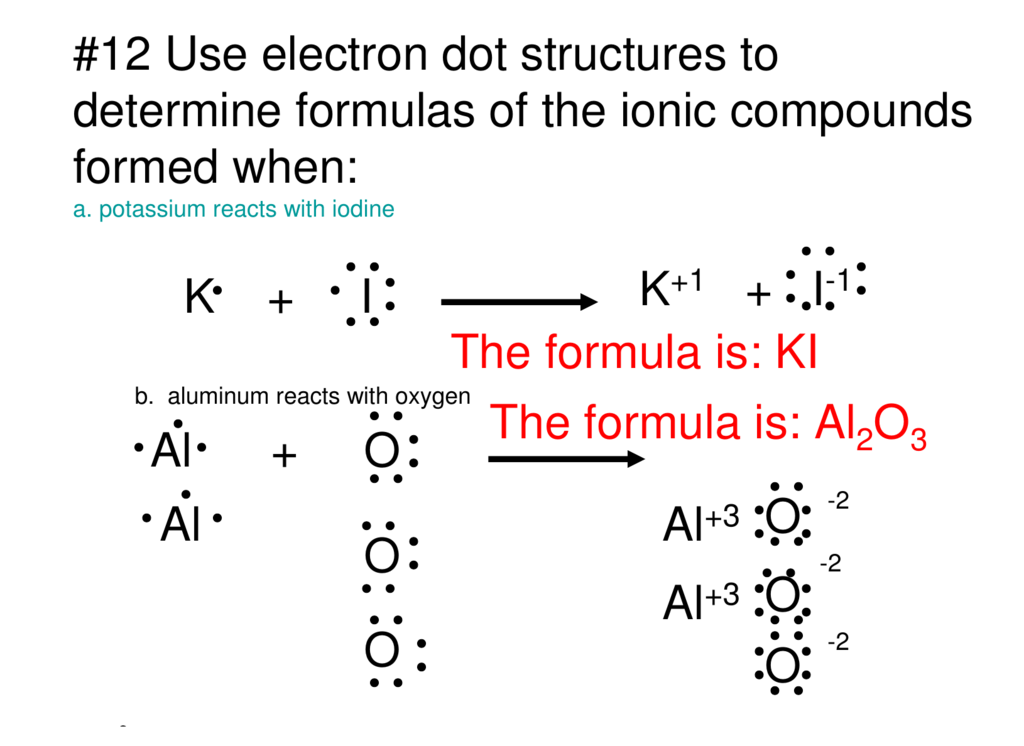
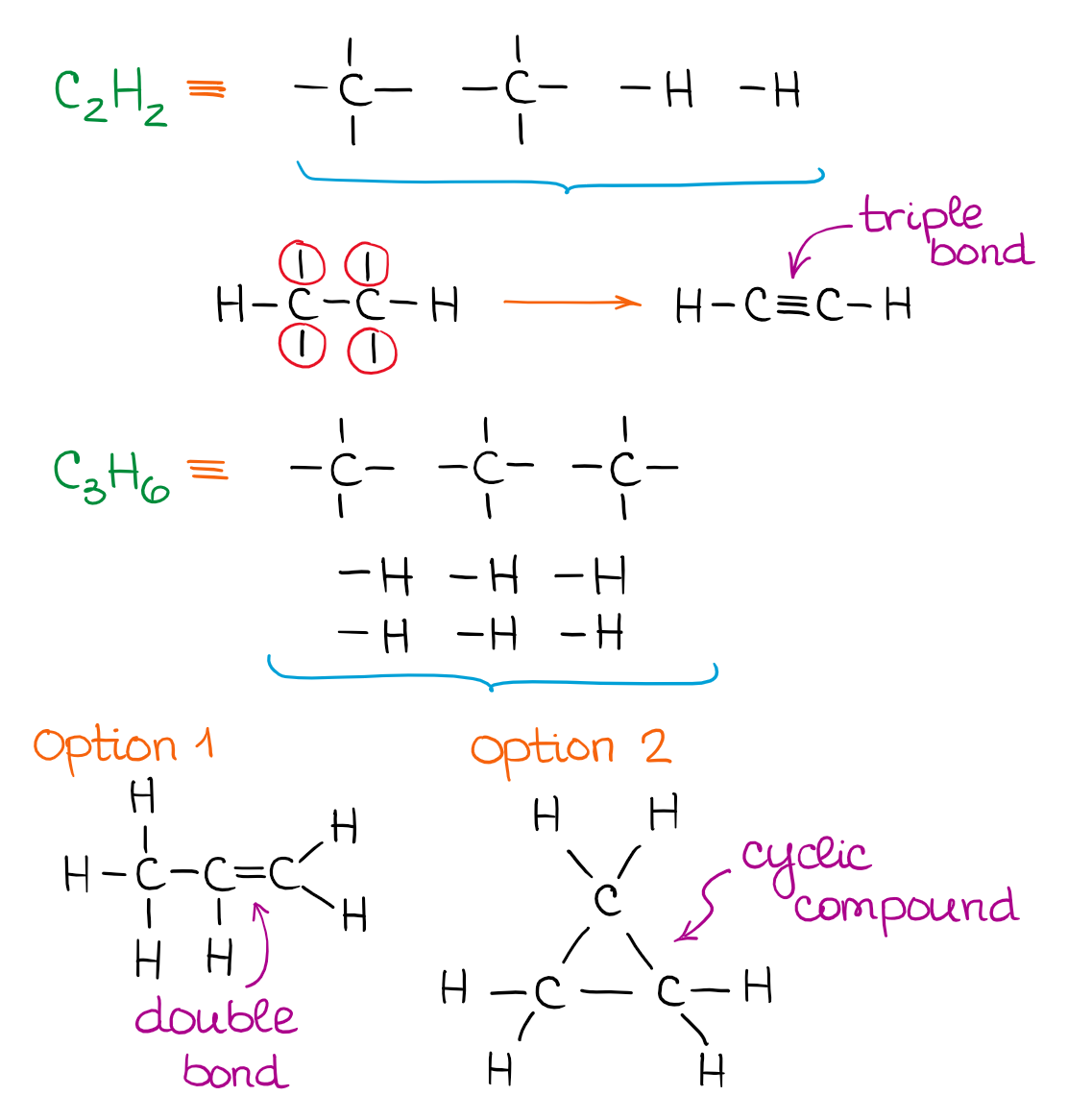

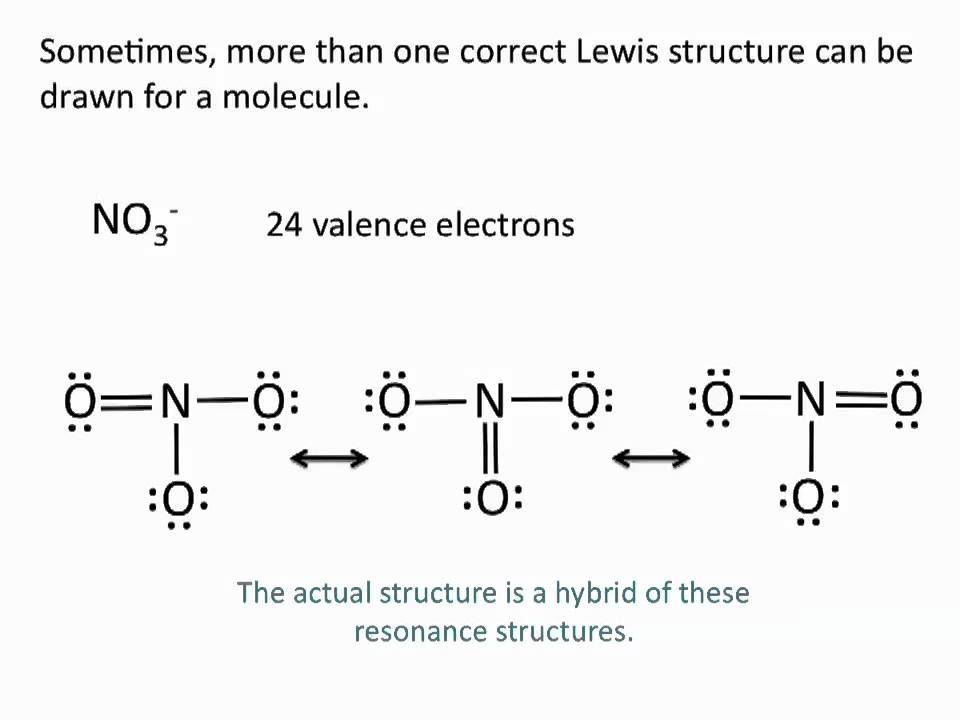
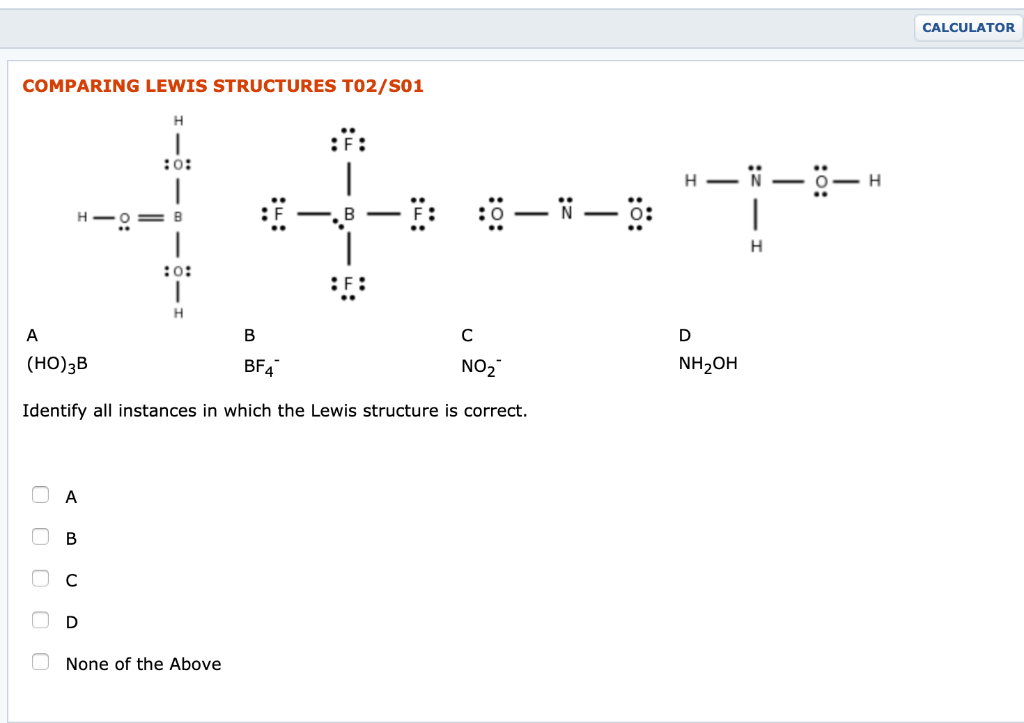



0 Response to "42 lewis dot diagram calculator"
Post a Comment