42 molecular orbital diagram h2-
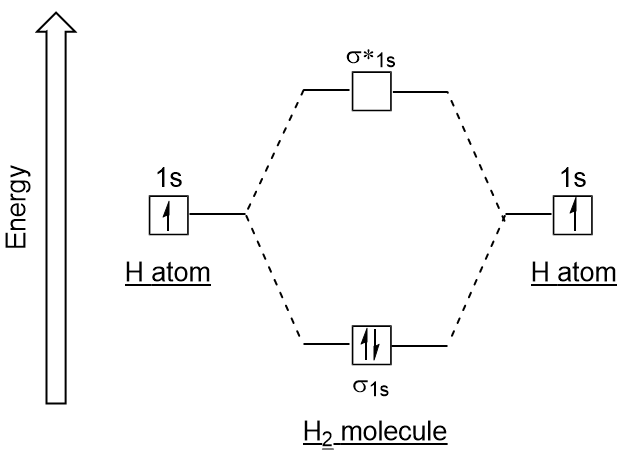
Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me ...
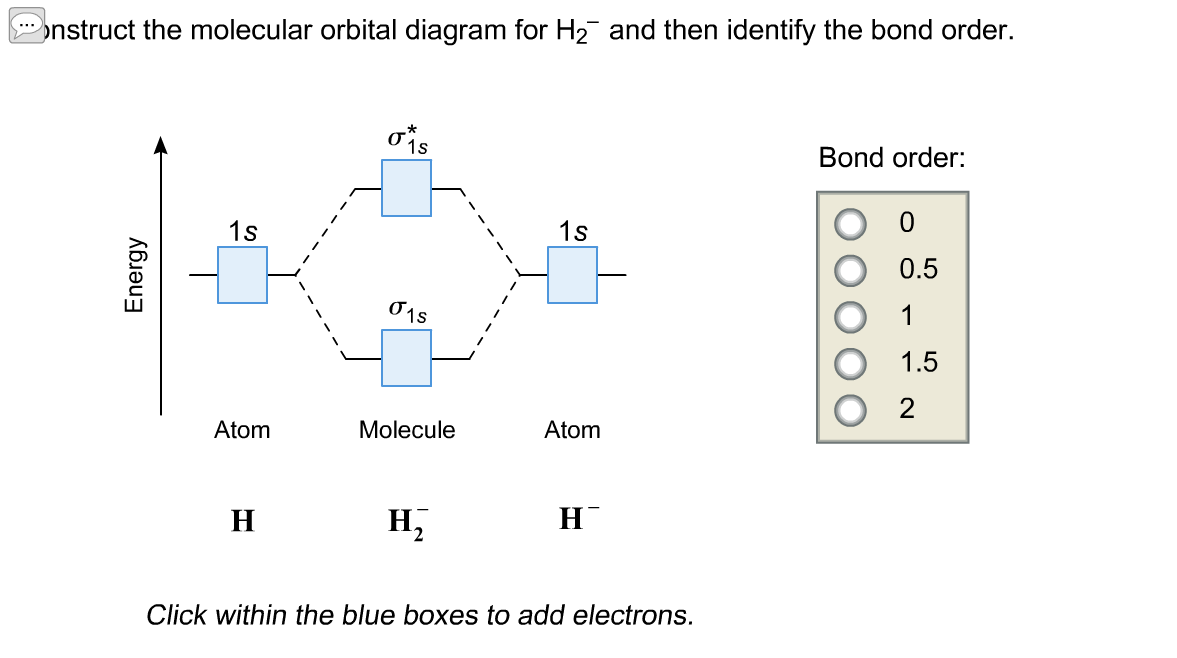
Now on adding one electron to H2, H2- is formed. So molecular orbital electronic configuration of H2- is σ1s² σ*1s¹ Bond order=1/2 [number of bonding electrons -number of antibonding electrons] bond Continue Reading Jasdeep Singh , Btech. from Lovely Professional University- LPU Answered 3 years ago Atomic no. Bond order 10 1 11. 1.5 12. 2 13 2.5
This video discusses how to draw the molecular orbital (MO) diagram for the H2(2+) molecule. The bond order of H2(2+) is calculated and the meaning of this n...
Molecular orbital diagram h2-
Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here.
a molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbital s (lcao) molecular orbital method in particular.the hydrogen molecule ion h2 + molecular orbital diagram s of diatomic molecules - chem …
Mo · Construct The Molecular Orbital Diagram For H2 And Then Identify The Bond Order. chemical bonding molecular orbitals of h2 and he2 as before the greater the number of these nodal planes the more the electrons that occupy the orbitals are excluded from the region between the nuclei and hence the higher the energy the resulting molecular ...
Molecular orbital diagram h2-.
14+ H2 Molecular Orbital Diagram. A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals.
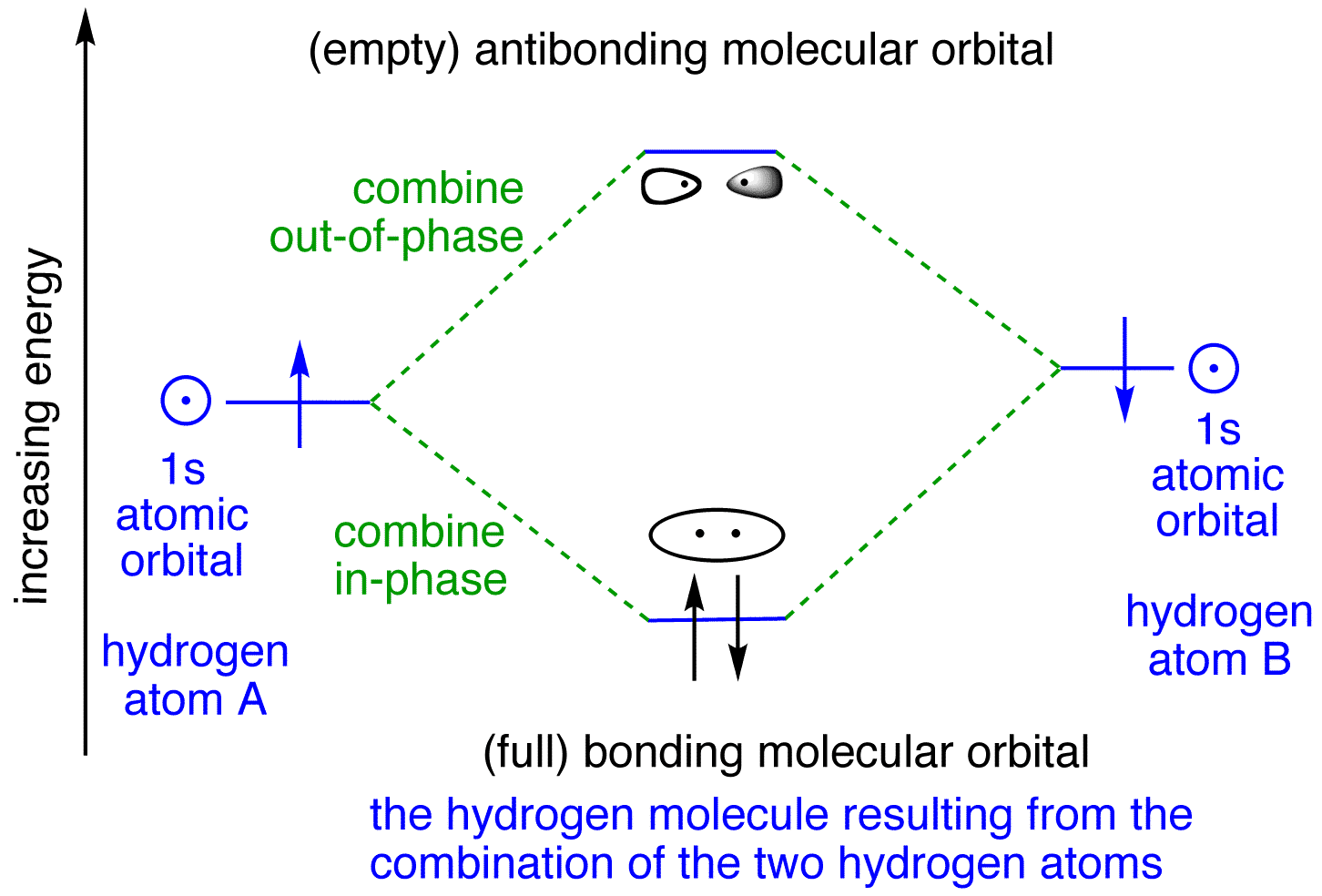
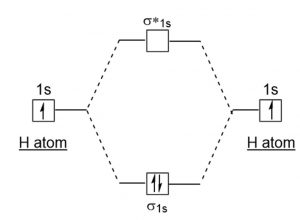
Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, H− 2 has three electrons while H+ 2 has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one σ1s and one σ* 1s MO by conservation of orbitals.
D.Construct the molecular orbital diagram for H2^+ and then identify the bond order. *need before 7:00; Question: A.Construct the molecular orbital diagram for H2^2+ and then identify the bond order. B.Construct the molecular orbital diagram for H2 and then identify the bond order.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.The Hydrogen Molecule Ion H2+Molecular Orbital Diagrams of Diatomic Molecules - Chem
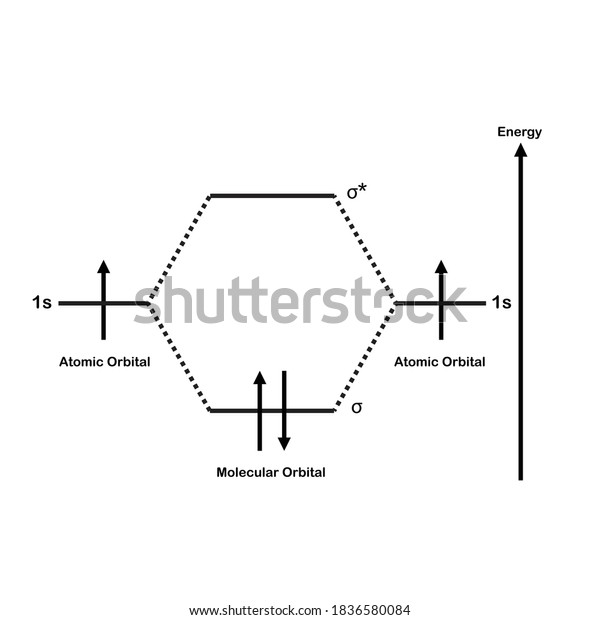
38 molecular orbital diagram of h2. Written By Chelsea P. Mariano Monday, November 8, 2021 Add Comment. Edit. 3 Feb 2021 — For H2, bond order = 1/2 (2-0) = 1, which means H2 has only one bond. The antibonding orbital is empty. Thus, H2 is a stable molecule. Again, in ...
Molecular orbital diagram h2. Because of their simplicity they have been extensively studied. Two superpositions of these two orbitals can be formed one by summing the orbitals and the other by taking their difference. Construct the molecular orbital diagram for h2 and then identify the bond order.
32846. Table of contents. Contributors and Attributions. Describe the hydrogen molecule in light of the following: H − H. H: H. Valence bond theory of H 2. Molecular orbital theory of H 2. Electron configuration of molecules.
Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...
Molecular Orbital Diagram. Molecular Orbital Diagram to basically describe what is molecular orbital and provide you with some example of it in diagram. Welcome to 101diagrams.com, the site that provide great resources of images for your education and knowledge about various kind of diagrams. Including the medical diagrams, mathematics diagrams ...
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
of the orbitals is specified. b. Molecular Orbital Picture We are now in a position to discuss the basic principles of the molecular orbital (MO) method, which is the foundation of the electronic structure theory of real molecules. The first step in any MO approach requires one to define an effective one electron Hamiltonian, hˆ eff. To this ...
Science. Chemistry. Chemistry questions and answers. Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes to add electrons. Question: Construct the molecular orbital diagram for H2- and then identify the bond order.
is called Molecular Orbital Theory. • MO theory assumes that the valence electrons of the atoms within a molecule become the valence electrons of the entire molecule. • Molecular orbitals are constructed by taking linear combinations of the valence orbitals of atoms within the molecule. For example, consider H2:
A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 .
Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic ... Spin‐orbitals of type 1 and 3 have the same symmetry, and therefore can "mix" (to give improved wavefunctions and energy eigenvalues): 1 ψψ αβ ...
the lowest energy, or bonding, molecular orbital, as shown in the figure below. This diagram suggests that the energy of an H2molecule is As a result, the H2molecule is more stable than a pair of isolated atoms. Using the Molecular Orbital Model to Explain Why Some Molecules Do Not Exist
Mo Diagram H2. molecular orbital diagram a molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory marcus va 100 primary volts 120 240 secondary volts 12 24. Construct The Molecular Orbital Diagram For H2- And Then Identify The Bond Order.
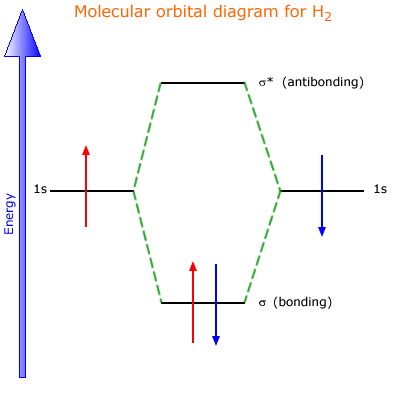
MO DIAGRAM FOR DIATOMIC HYDROGEN MOLECULE & ION Now, let us draw the MO diagram for the H2 neutral molecule. Each hydrogen contributes one electron, which therefore fills the lower-in-energy σ1s bonding orbital. Add one electron, and you will get H− 2, thus giving an electron in the antibonding σ* 1s MO. MO ELECTRON CONFIGURATIONS
the molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the h 2 molecule is shown in figure on either side of the central ladder are shown the energies of the 1 s orbitals of atoms a and b, and the central two-rung ladder shows the energies of the bonding and antibonding.the …
Construct the molecular orbital diagram for h2 and then identify the bond order. Each hydrogen atom contributes one electron and thus h2 has three electrons while h2 has one. Discussed in this video are. Draw mo energy diagrams for the molecular ions h2 and h2. Construct the molecular orbital diagram for h2.
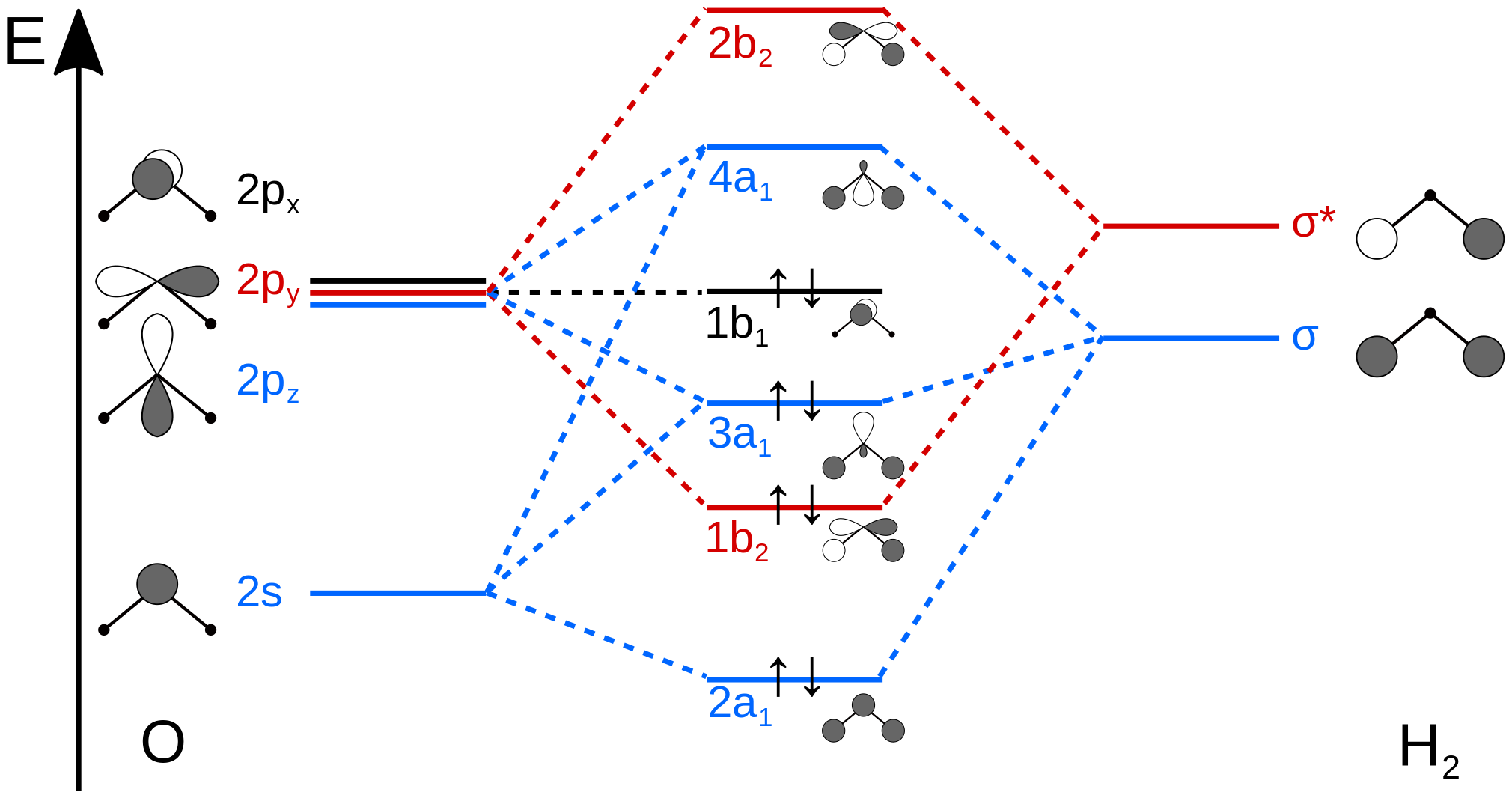
Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
We can therefore use a molecular orbital energy-level diagram and the calculated bond order to predict the relative stability of species such as H 2+. With a bond order of only 1/2 the bond in H 2+ should be weaker than in the H 2 molecule, and the H-H bond should be longer.
The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure 13.























0 Response to "42 molecular orbital diagram h2-"
Post a Comment