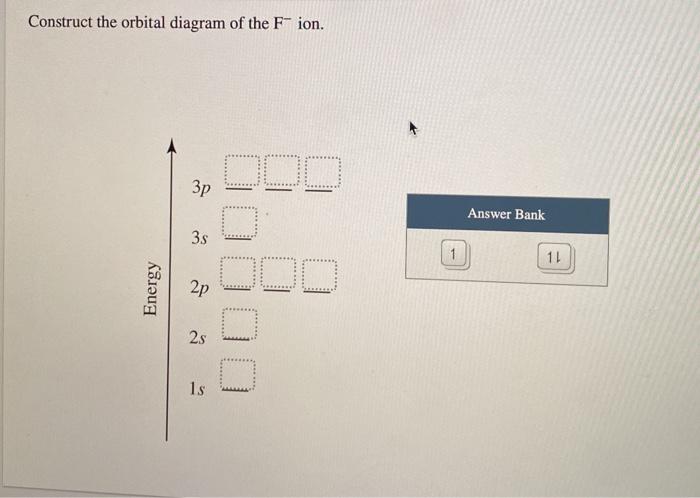
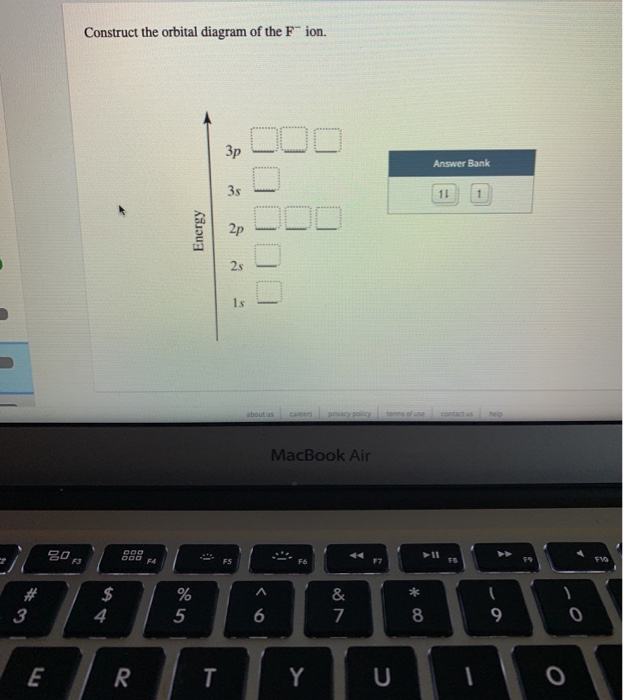
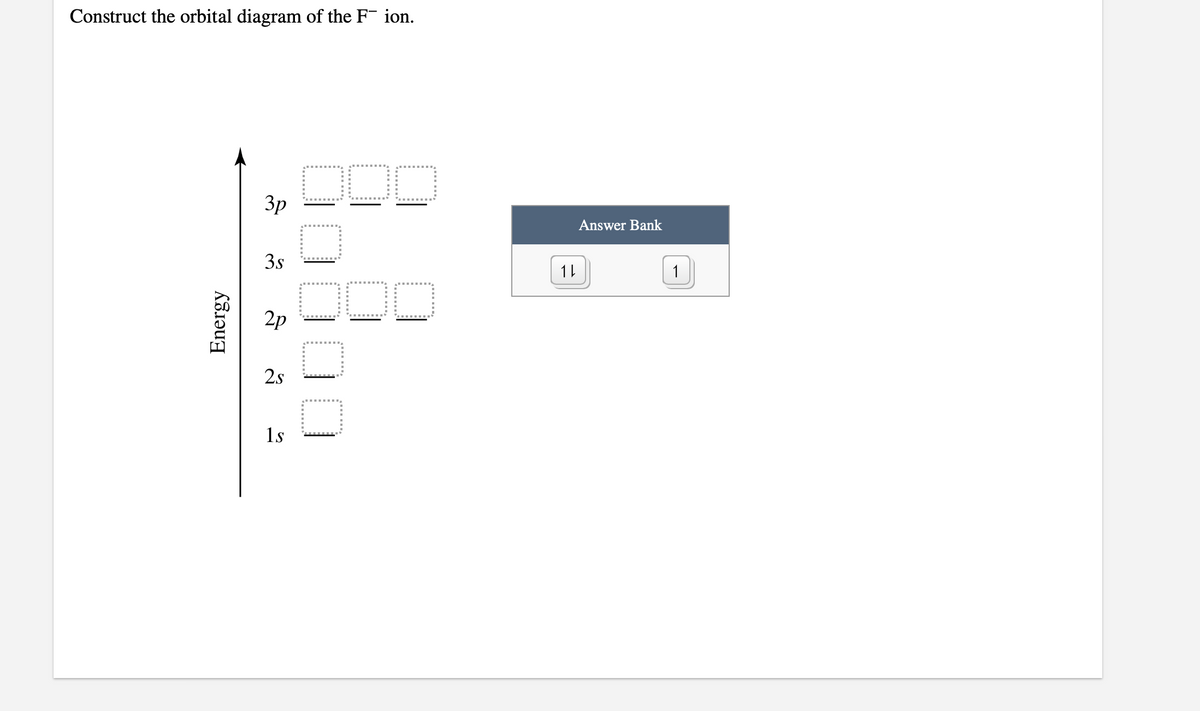
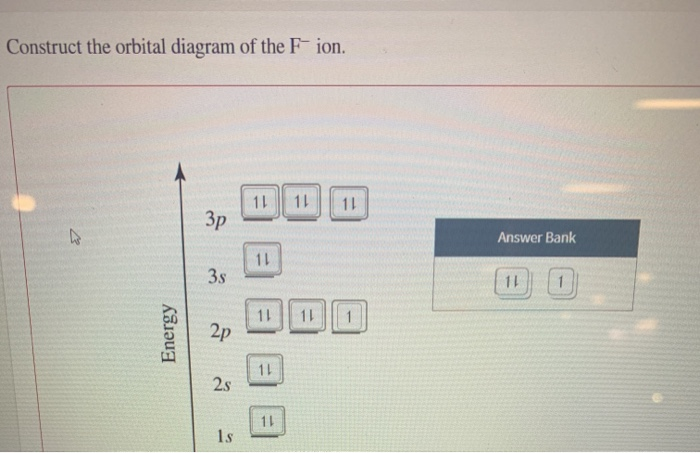
43 construct the orbital diagram of the f– ion.
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
The electron previously filled in d-orbital of the free metal ion can now be considered as distributed between t2g * and e g *. Hence, the crystal field splitting Δ o decreases when ligand to metal bonding takes place. The overall molecular orbital energy level diagram for this type of π-bonding in octahedral complexes can be shown as:
Question: Construct The Orbital Diagram Of The F^- Ion. A Neutral Fluorine Atom Has 9 Electrons. How Many Electrons Does A F^- Ion Have? This problem has been solved! See the answer. Show transcribed image text. Videos. Step-by-step answer 05:59 0 0. Expert Answer 100% (22 ratings) ...

Construct the orbital diagram of the f– ion.
This photo about: Construct the orbital Diagram Of the F- Ion, entitled as Molecules Free Full Text Construct The Orbital Diagram Of The F- Ion - also describes Molecules Free Full Text and labeled as: ], with resolution 3361px x 2298px
Construct the orbital diagram of the F^- ion. Subject: Chemistry Price: 2.85 Bought 3 Share With. Construct the orbital diagram of the F^- ion. A neutral fluorine atom has 9 electrons.
Orbital Diagram Of Ti2+. Answer to Construct the orbital diagram of each atom or ion. Ti Ti2+ Ti4+. Figure A vertical orbital diagram for the Li ground state. .. Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons . When filling degenerate orbitals, electrons fill them singly first, with parallel spins is ...
Construct the orbital diagram of the f– ion..
FREE Expert Solution. We are asked to construct the orbital diagram of the F - ion. F → 9 electrons. Negative charge adds 1 electron more. F - → 10 electrons. 97% (333 ratings)
Fluorine(F) is the 9th element in the periodic table and the first element in group-17. The standard atomic mass of fluorine is 18.998403 and its symbol is 'F'. The period of fluorine is 2 and it is a p-block element. The electron configuration of fluorine(F) and the orbital diagram is the main topic of this article.
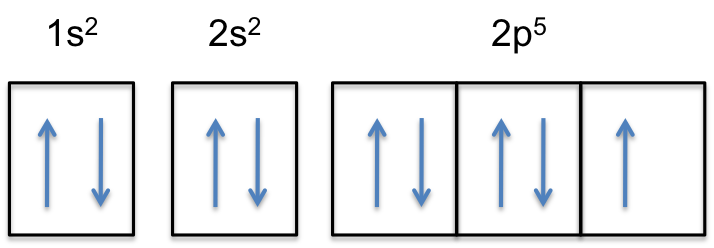
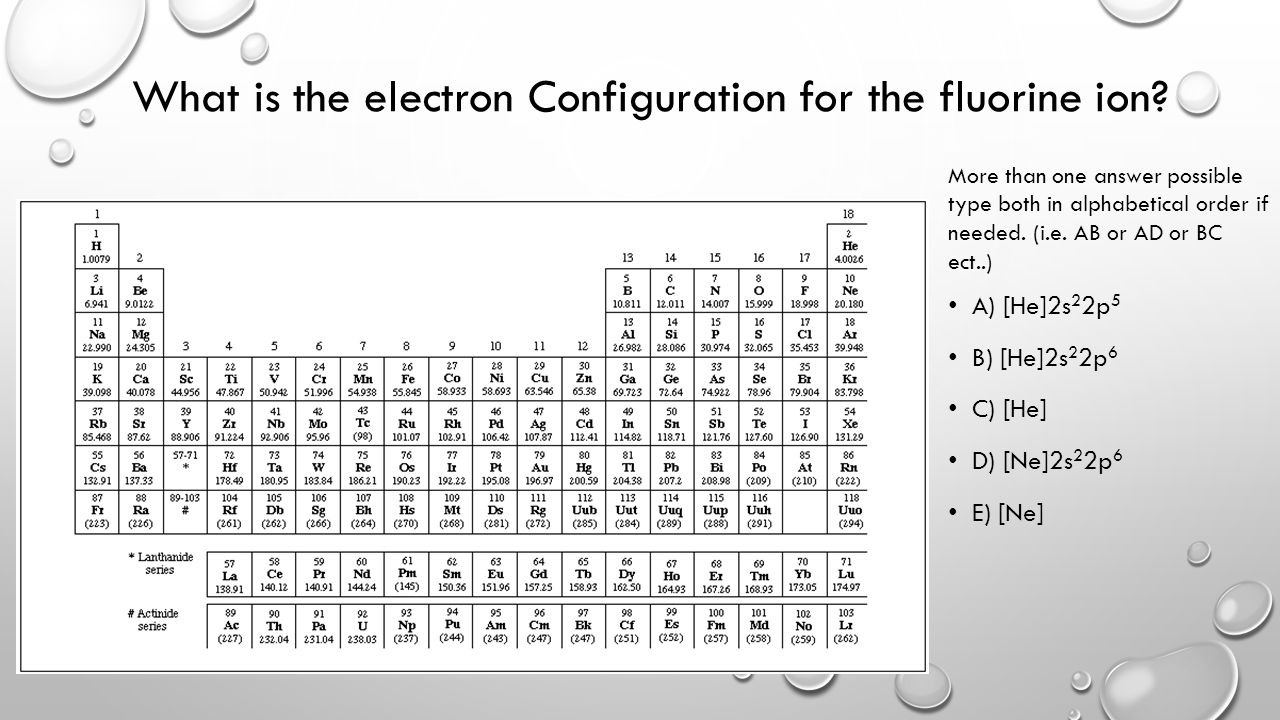
Jun 23, 2016 · "F"^(-): 1s^2 2s^2 2p^6 A good starting point for when you must find the electron configuration of an ion is the electron configuration of the neutral atom. In your case, you must find the electron configuration of the fluoride anion, "F"^(-), so start by writing the electron configuration of a neutral fluorine atom, "F". Fluorine is located in period 2, group 17 of the periodic table and has ...
The s-orbital does not support the Hund principle. The electron configuration of oxygen in Hund's principle is 1s 2 2s 2 2p x2 2p y1 2p z1. The electron configuration of oxygen in excited state is O* (8) = 1s 2 2s 2 2p x2 2p y1 2p z1. The last orbital of oxygen is 'p'. And unpaired electrons exist in its last p-orbital.
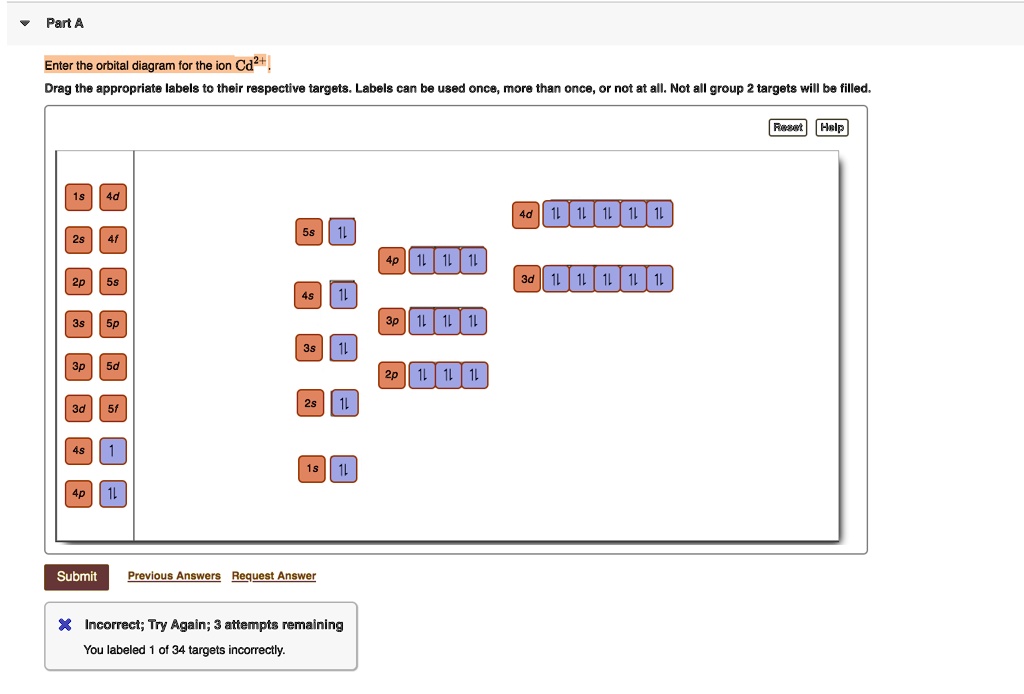
Part B. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the lowest energy orbitals. Click within an orbital to add electrons. Part C. Draw the orbital diagram for the ion N3−.
Answer (1 of 3): In O2 2+, there is 14 electrons. So, it's MOT is comparable to N[code ]2[/code] & the MOT diagram will look like this :
1 ½ e. 2 Construct the molecular orbital diagram for H 2- a What charge would be needed on F 2 to. Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. Figure \(\PageIndex{1}\): Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1s Atomic Orbitals.
Fluorine is the ninth element with a total of 9 electrons. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for F go in the 2s orbital. The remaining five electrons will go in the 2p orbital. Therefore the F electron configuration will ...
Is this the correct atomic orbital diagram for a calcium (2+) ion? Please indicate "true" or "false" accordingly on your Google quiz form.
Relative AO Energies in MO Diagrams Use AO energies to draw MO diagram to scale (more or less). H He Li Be B C N O F Ne B C N O F Ne Na Mg Al Si P S Cl Ar Al Si P S Cl Ar 1s 2s 2p 3s 3p -19.4 eV -15.8 eV -32.4 eV -10.7 eV
Electron Configuration For Nitrogen Ion. The atomic number of nitrogen is 7, the element nitrogen was discovered by a Scottish physician, Danial Rutherford. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element Nitrogen. ... When we talk about the orbital ...
Answer (1 of 4): The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To find the bond order, add th...
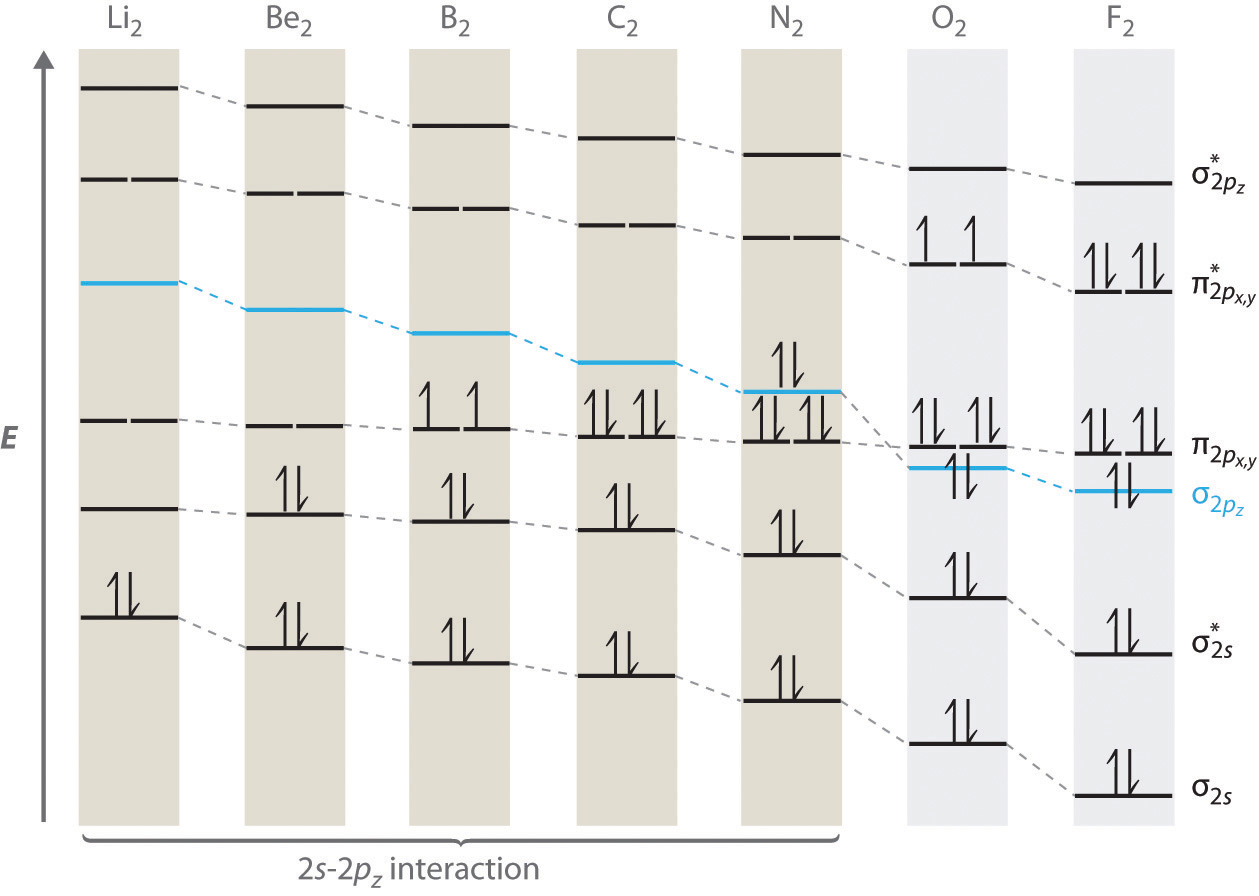
Draw molecular orbital diagram for F 2 molecule. Also, gives its electronic configuration, bond order and magnetic property. Hint: The Molecular Orbital Theory (MOT) explains the formation of the molecule in a better way than Valence Bond Theory (VBT). The bond order calculations are feasible using MOT and so is the description of electronic ...
This problem has been solved! See the answer. See the answer See the answer done loading. Construct the orbital diagram of the F– ion. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (22 ratings)
Construct the orbital diagram (using the arrow-in-box notation) for iron, showing the electrons in the \(n = 3\) and \(n = 4\) energy levels only and label each sub-level on the diagram. [1] e. ... Draw the abbreviated orbital diagram for the iron ion in [Fe ...
Feb 23, 2018 · Orbital diagram of f ion. The remaining five electrons will go in the 2p orbital. The following is the diagram for the neutral oxygen. Electron configurations orbital diagrams. An orbital diagram naturally leads to the writing of an electron configuration. Ion electron confugurations.
degenerate antibonding molecular orbital (t* 1u) set. The φ5 and φ6 composite orbital set of eg symmetry interacts with eg orbital set of metal ions and produces doubly-degenerate bonding (eg) and doubly-degenerate antibonding molecular orbital (e* g) set. The t2g orbital set of the metal center remains non-bonding in nature.
[Kr] 4d Draw the orbital filled diagram for this ion. What is an example of a balanced chemical reaction for the formation of Cadmium hydroxide?.Jul 21, · Best Answer: orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 you just have to fill the "boxes" with arrows, s orbital has only one box with two arrows ...
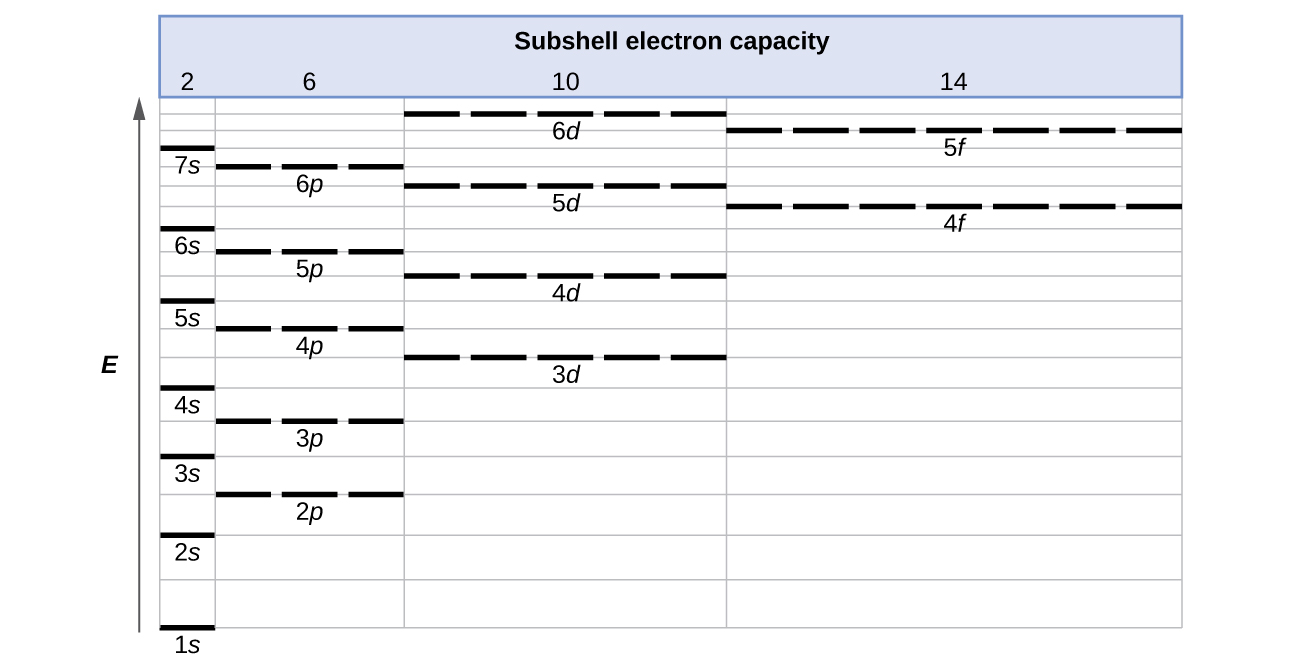
The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital. Orbital diagrams use the same basic format, but instead of numbers for the electrons, they ...
Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...
Molecular orbital diagram of N 2 − is shown below: This picture shows the molecular orbital diagram of N 2 − . Orbitals represented by ∗ are antibonding orbitals and the orbitals without ∗ are bonding orbitals. Bond order can be calculated by the formula: Bond order = bonding electrons - antibonding electrons 2.
Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...
Construct the orbital Diagram Of the F– Ion. construct construct the orbital diagram the f– ion chem 120a november 8 2005 fall 2004 8 00 – 9 20 am exam ii name prepare a molecular orbital energy level diagram for no construct a solved construct the orbital diagram the f ion a ne answer to construct the orbital diagram of the f ion a neutral fluorine atom has 9 electrons how many ...
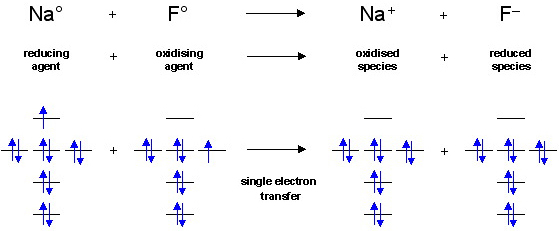
F^- : 1s^2 2s^2 2p^6 alternatively: F^- : [Ne] Elemental Fluorine has an electron configuration of 1s^2 2s^2 2p^5 and needs 1 more electron to complete its 2p orbital which it will acquire in formation of the fluoride ion. Thus it gains an electron when forming the fluoride ion, and becomes isoelectronic to neon.
Draw the molecular orbital diagram of N 2. Also find its bond order and magnetic character? Asked by Topperlearning User | 13th Jun, 2016, 02:45: PM. Expert Answer: Molecular orbital diagram of N 2 BO = [Nb-Na] = [10-4] = 3 Since all the electrons in nitrogen are paired, it is diamagnetic molecule.
Nov 16, 2021 · Ion electron confugurat ion s. A neutral fluorine atom has 9 electrons. Show transcribed image text construct the orbital diagram of the f ion. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbital s in order of increasing energy, starting at the bottom with the. Answer to Write orbital diagram for Co2+.












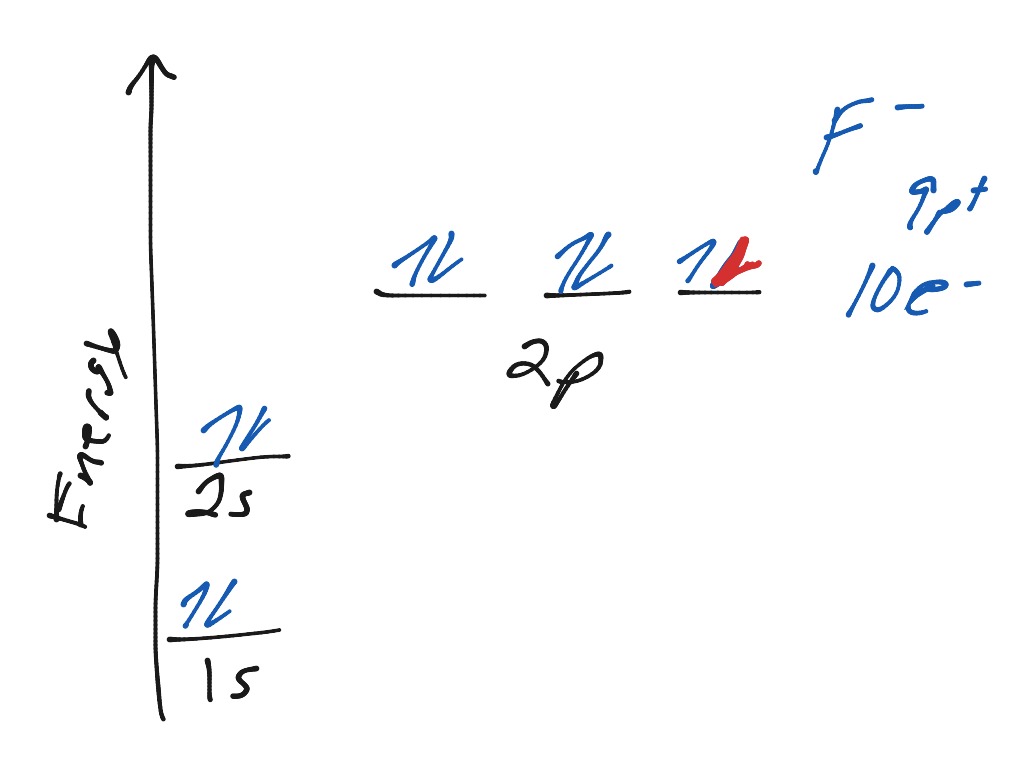












0 Response to "43 construct the orbital diagram of the f– ion."
Post a Comment