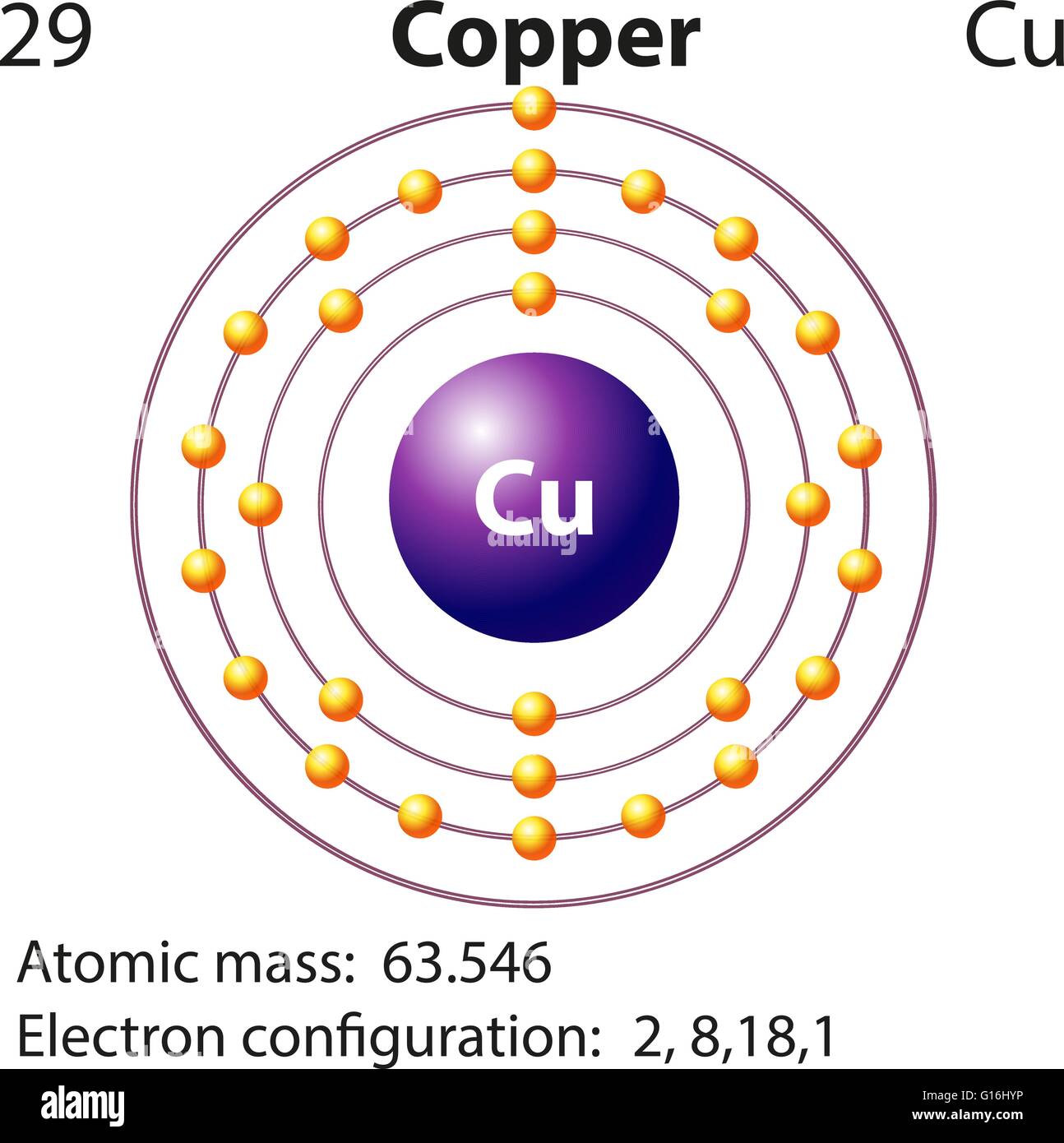
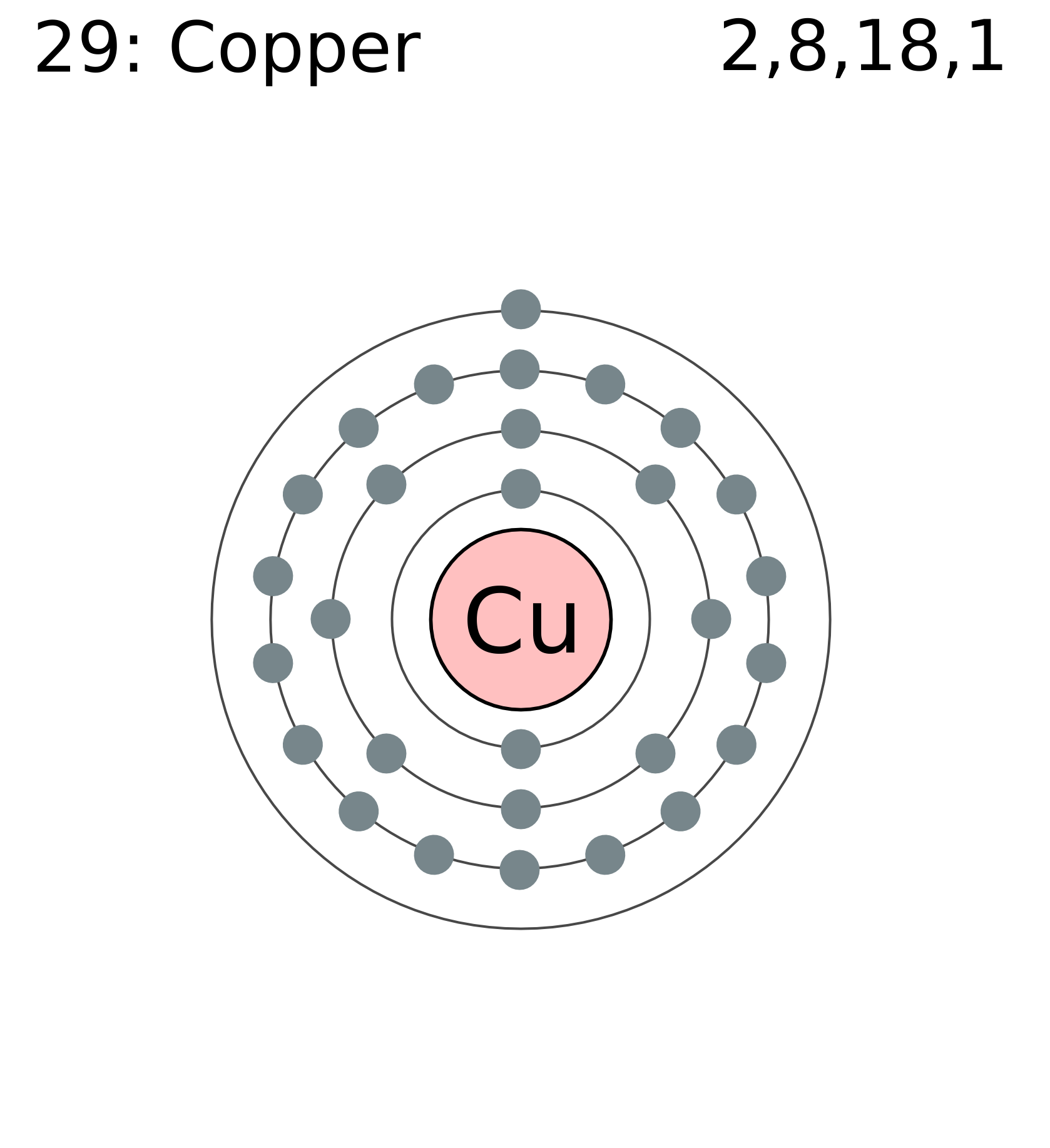
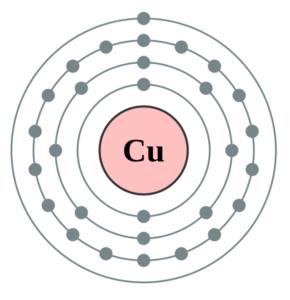
44 copper electron dot diagram
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
Copper is obtained by smelting leaching and by electrolysis. Orbital diagrams noble gas configuration lewis dot diagrams. Gilbert newton lewis surrounded by pairs of electrons. Lewis dot diagrams lewis dot diagrams are a way to represent the valence electrons in an atom. In copper ii fluoride the cation is the copper ii ion and the anion.
Hence no electron stays in this band. The valence electrons, while going to the conduction band, pass through this. The forbidden energy gap if greater, means that the valence band electrons are tightly bound to the nucleus. Now, in order to push the electrons out of the valence band, some external energy is required, which would be equal to the forbidden energy gap. The following …

Copper electron dot diagram
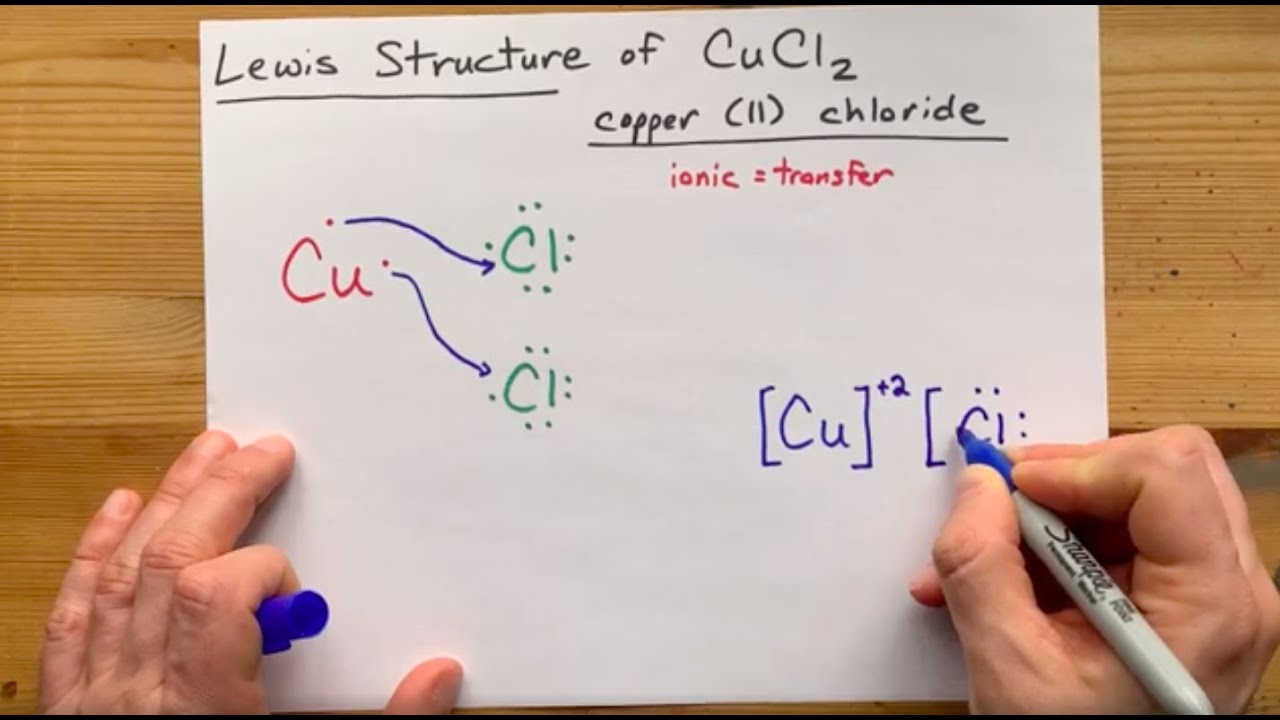
Sulfur wants to accept 2 electrons for a full valence shell (see it's column in the periodic table). · So, copper donates 2 electrons to sulfur to form an ionic ...1 answer · 3 votes: Copper wants to donate 2 electrons for a full valence shell (see it’s column in the ...
1. Draw an “electron dot” diagram showing the first 18 elements in the periodic table. 2. Explain how the electron dot diagram is similar for families in the periodic table. 3. Draw an electron dot diagram showing the formation of ions and ionic compounds. 4. Explain how hydrogen can be considered as behaving like a metal or a nonmetal.
Which electron configuration represents the electrons of an atom of neon in an excited state
Copper electron dot diagram.
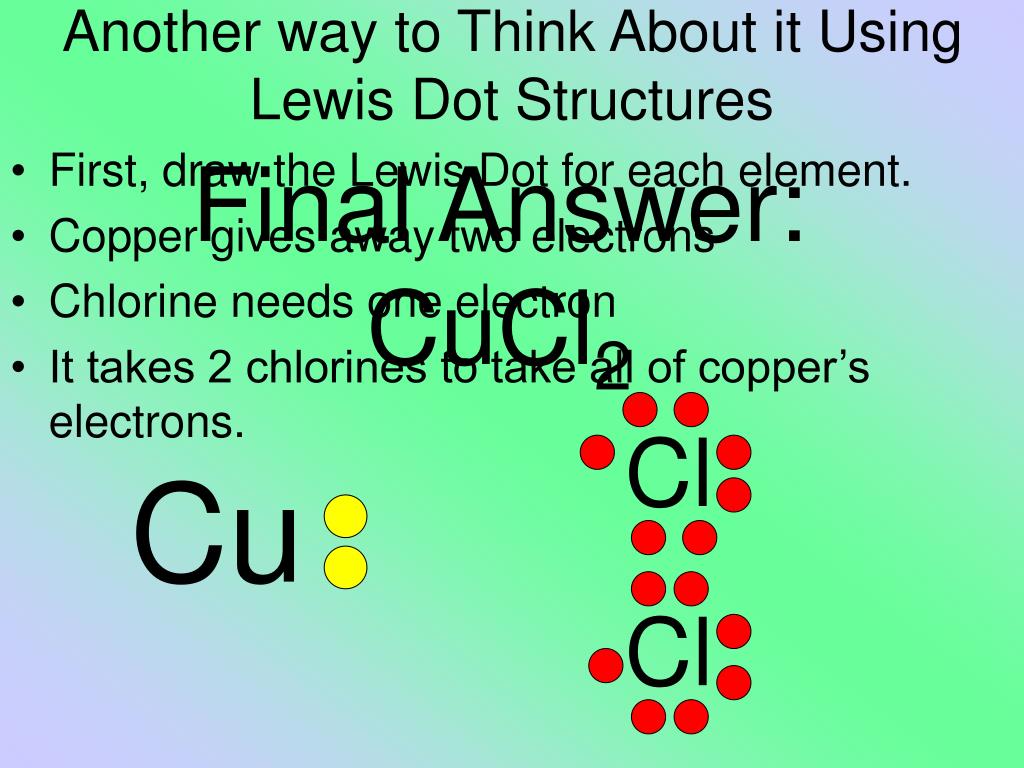
12.12.2017 · e) In the box below, draw the Lewis electron-dot structure for the compound formed from magnesium and chlorine. [ Include any charges or partial charges.] (1 pt.) 32) Explain, in terms of electronegativity, why an H-F bond is expected to be more polar than an H-I bond. (2 pts.) BONUS Questions – 1 pt. each 33) Given the reaction: H2 + Cl 2 2HCl
A step-by-step explanation of how to draw the Cu(OH)2 Lewis Dot Structure.For Cu(OH)2 we have an ionic compound and we need to take that into account when we...
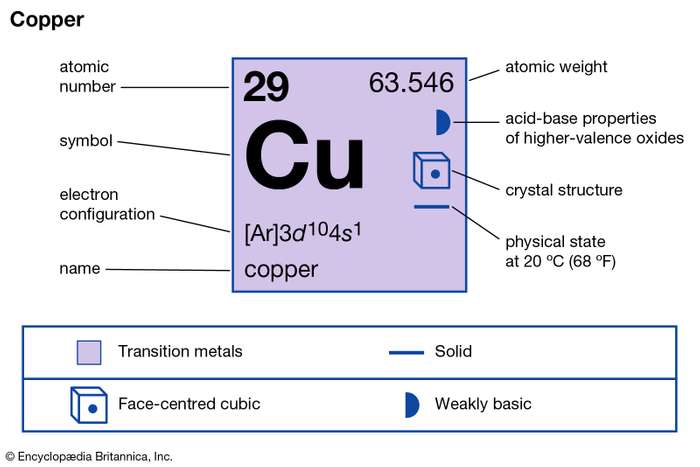
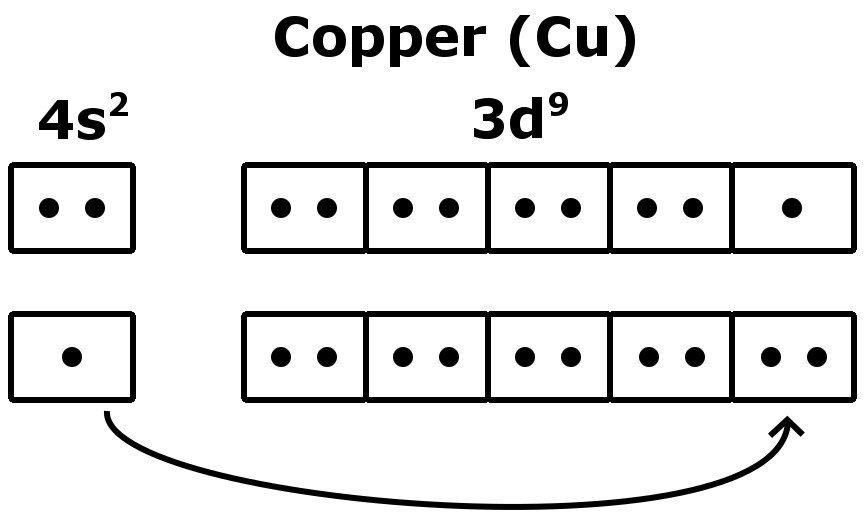
Therefore the expected electron configuration for Copper will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 9. Note that when writing the electron configuration for an atom like Cu, the 3d is usually written before the 4s.
01.09.2021 · Bohr Diagram For Phosphorus. Electron Configuration Notation: shows the arrangment of electrons around the nucleus of an atom. – helps chemist understanding how elements form chemical. Neon Atom Model, 5th Grade Science Projects, 8th copper bohr diagram wedocable – 28 images – copper element protons and.
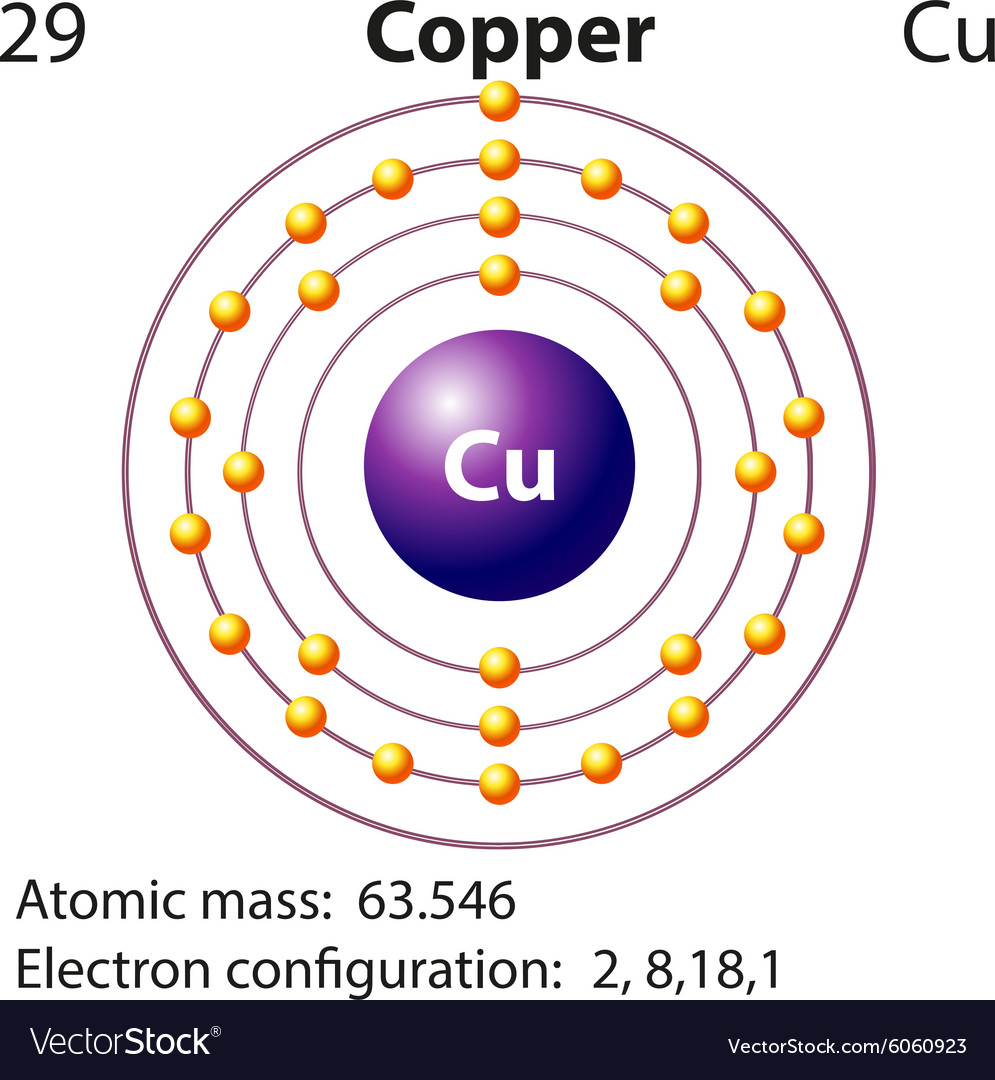
A. Write the electron configuration and orbital diagrams of Be2+, Al3+, Ca.B. What is a Lewis dot symbol ? To what elements does the symbol mainly apply?for ...1 answer · 0 votes: Chemical symbol: Cu Number of electrons: 29 1 on the valence shell
Lewis Dot Structures. to show the valance electrons of an element as dots. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons. Element: Bohr Diagram; Group Number (PT) # of Valance Electrons; Lewis Dot Structure; Calcium. Carbon. Hydrogen. Helium. Oxygen. Fluorine. Neon. Sodium. Aluminum. Determining …
Write the electron configuration for zinc. 15. Fill in the electron configuration diagram for the copper(I) ion. Copper atom Cu 3d 4s 3p 3s 2p 2s 1s Energy level Copper(I) ion Cu Formation of Anions 16 . Atoms of most nonmetallic elements achieve noble-gas electron configurations by gaining electrons to become , or negatively charged ions. 17.
The copper ion exist in two possible oxidation state and these are Cu+ and Cu2+ C u 2 + . What is the correct Lewis dot diagram? A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
03.12.2021 · Dot Diagram Practice for Covalent Compounds (multiple copies) Ch. 7 Test Review - optional assignment ; LABS. Electron Dot Diagram Lab for Covalent Compounds with covalent data table and example answer to data table entry 1; Molecular Model Building Lab with data table - (three periods) Molecular Model Building Lab with shorter data table (two ...
3. Electron Dot shows only the valence (outer energy level) electrons . . Ex. :Oxygen atom . O . 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
A step-by-step explanation of how to draw the CuSO4 Lewis Dot Structure.For CuSO4 we have an ionic compound and we need to take that into account when we dra...
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more …
What is the Lewis dot diagram for copper? Organic Chemistry Lewis Structures and Bonding Lewis Dot Diagram. 1 Answer Karen L. Aug 2, 2016 Chemical symbol: Cu Number of electrons: 29 1 on the valence shell. Answer link ... What is the electron dot diagram for carbon?
The lewis structure for an element or ion can be drawn by representing the electrons of the valence shell as dots. The octet of the atom should be kept in mind ...1 answer · Top answer: The atomic number of Cu=29 The electronic configuration of its valence shell =3d10,4s13d10,4s1 Its lewis dot structure can be drawn as...
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1# C #1s^2 2s^2 2p^2# N #1s^2 2s^2 2p^3# O #1s^2 2s^2 2p^4# F #1s^2 2s^2 …






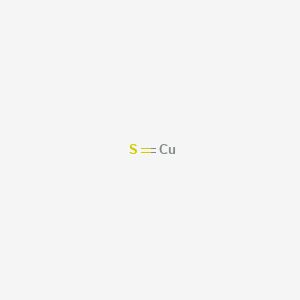


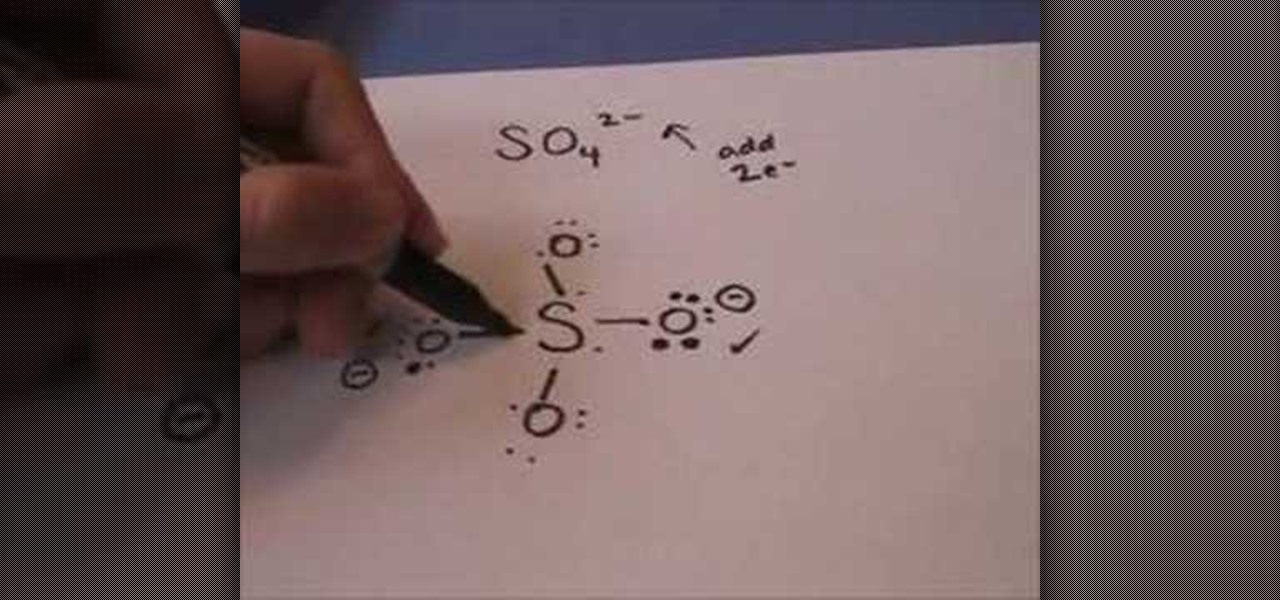



:max_bytes(150000):strip_icc()/Copper-58b602153df78cdcd83d352e.jpg)
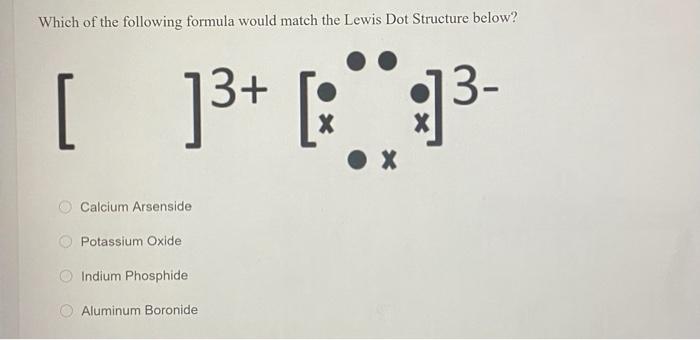
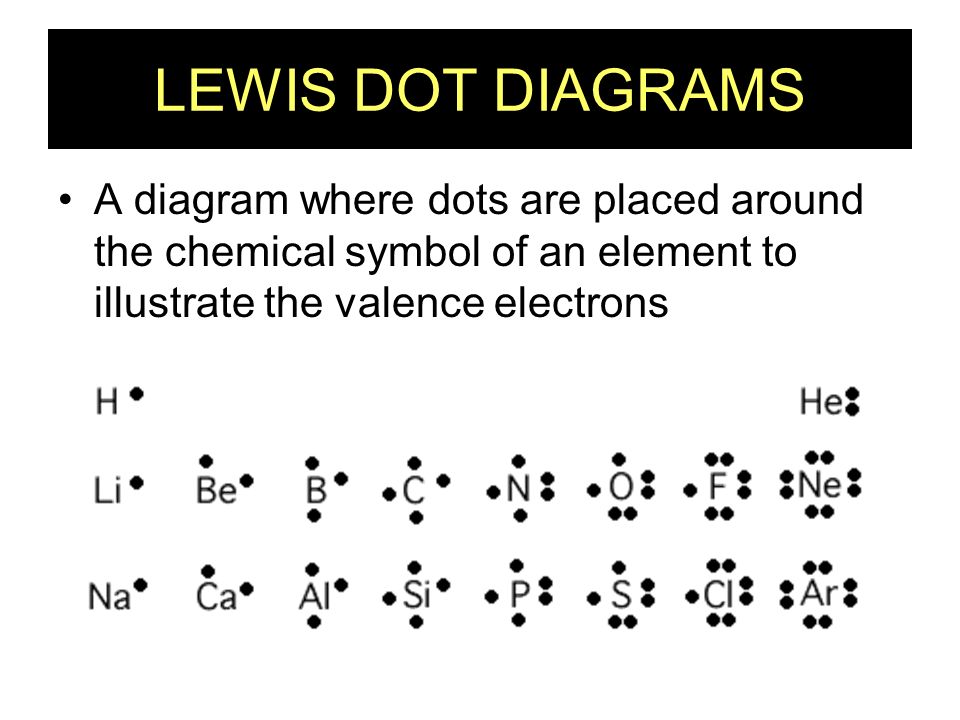
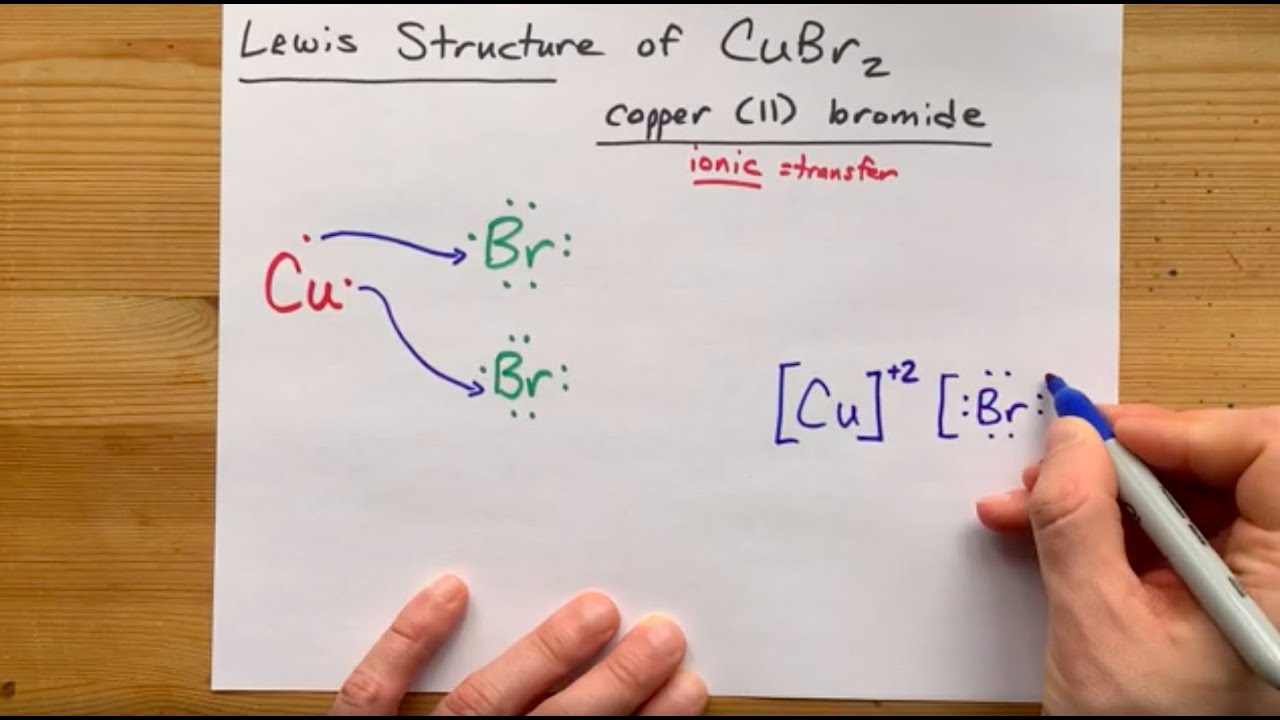

![a) Structures of the copper (Cu) complexes: [Cu(H2O)6] 2+ ...](https://www.researchgate.net/profile/Ivan-Shtepliuk/publication/339362935/figure/fig2/AS:860303357116417@1582123712768/a-Structures-of-the-copper-Cu-complexes-CuH2O6-2-CuH2O6-1-CuH2O6-0.jpg)



0 Response to "44 copper electron dot diagram"
Post a Comment