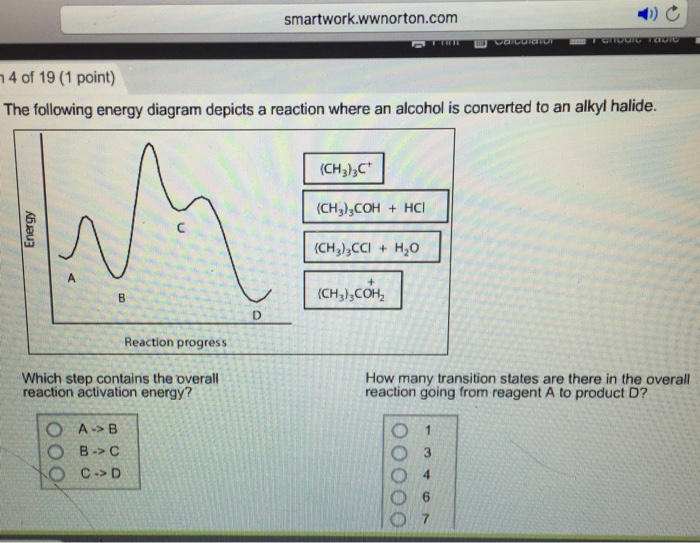
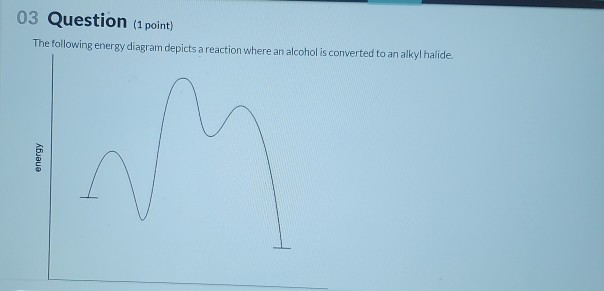
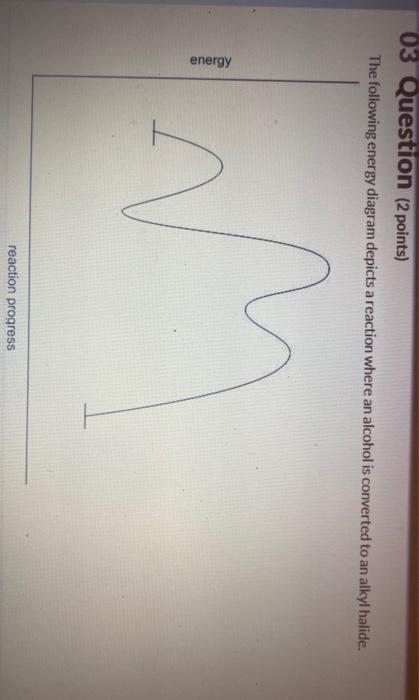
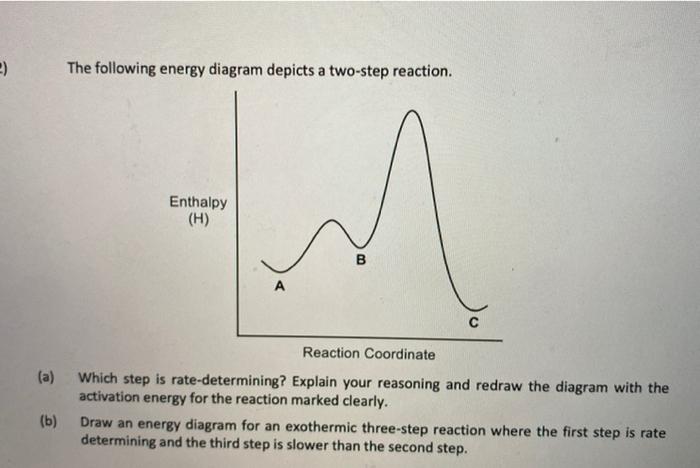
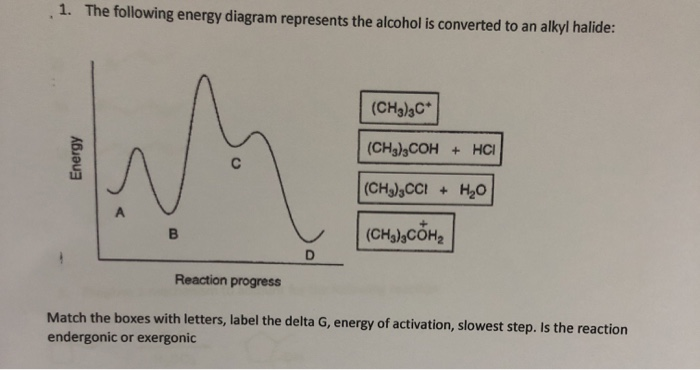
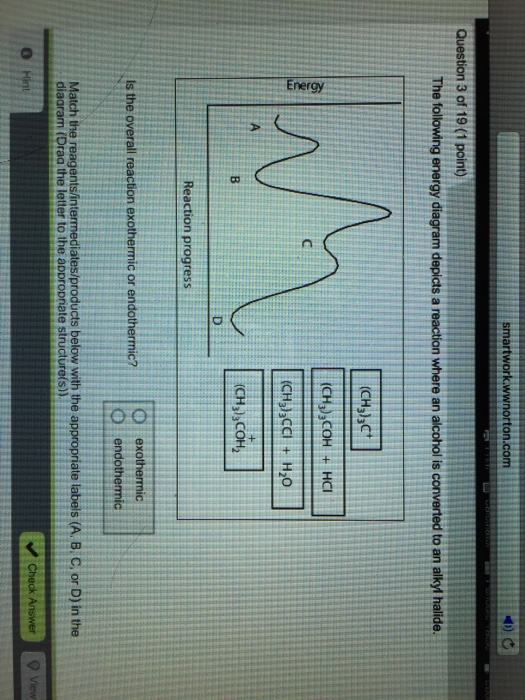
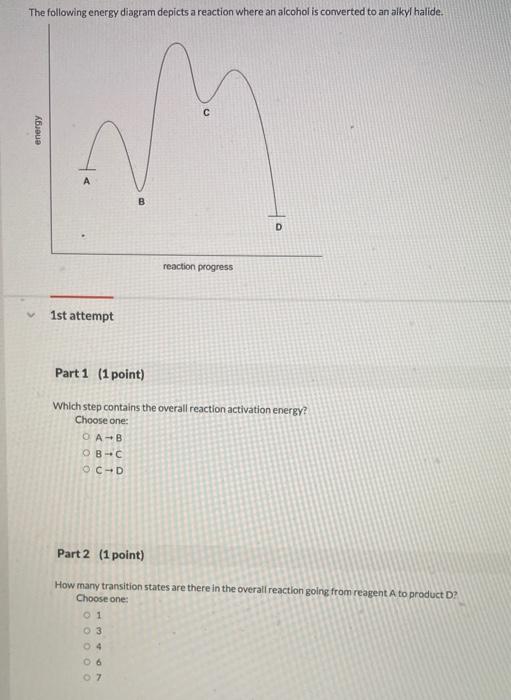
44 the following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide
The alkyl hydrogen sulfate can be converted to an alcohol by boiling in water. This proceeds usually by S N 1 substitution where water is the nucleophile and bisulfate is the leaving group. The product has the same regiochemistry as an alcohol formed by direct hydration of the same alkene. (Markovnikov orientation). Nonpolar (Hydrophobic) Amino Acids. The nonpolar amino acids can largely be subdivided into two more specific classes, the aliphatic amino acids and the aromatic amino acids. The aliphatic amino acids (glycine, alanine, valine, leucine, isoleucine, and proline) typically contain branched hydrocarbon chains with the simplest being glycine to the more complicated structures of leucine and valine.
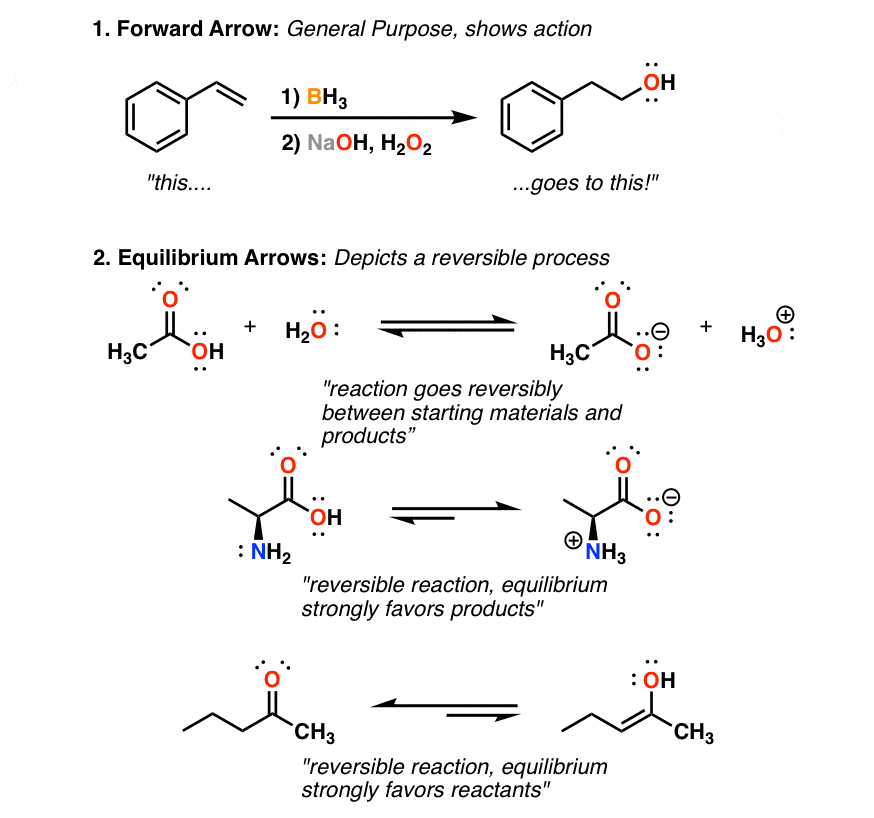
The S N 2 Reaction Notes: In the SN2 reaction, the nucleophile attacks from the most δ+ region: behind the leaving group. This is called a back-side attack. This back-side attack causes an inversion (study the previous slide): after the leaving group leaves, the other substituents shift to make room for the newly-bonded nucleophile, changing the stereochemistry of the molecule.
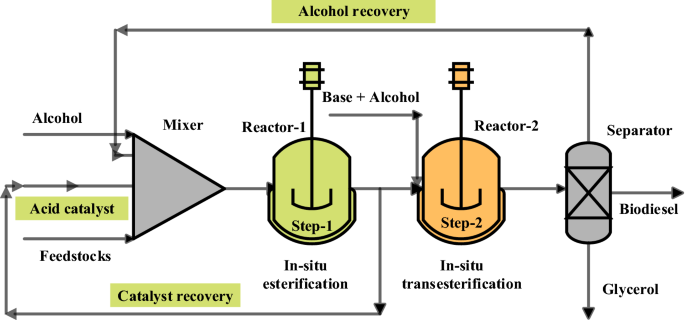
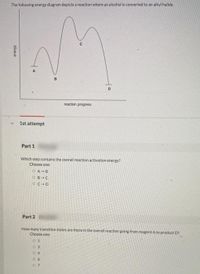
The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide
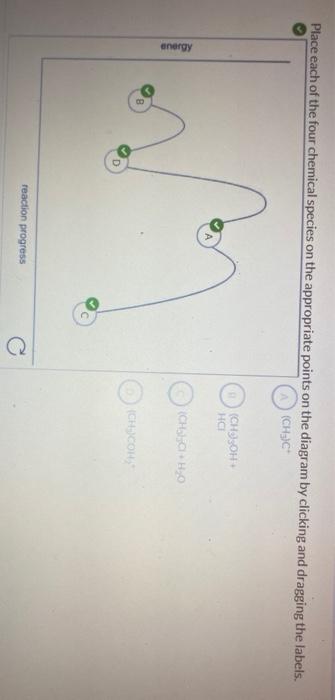
Acid catalyzed dehydration of secondary / tertiary alcohols. We'll take a look at a mechanism involving solvolysis during an E1 reaction of Propanol in Sulfuric Acid.. Step 1: The OH group on the pentanol is hydrated by H 2 SO 4.This allows the OH to become an H 2 O, which is a better leaving group.; Step 2: Once the OH has been hydrated, the H 2 O molecule leaves, taking its electrons with it. 5. Arrange the following alkyl halides in order of decreasing boiling point. 6. Predict the solvent with great alkyl halide solubility. a) water or hexane. b) water or 1-octanol. c) water or benzene. d) water or acetone. Solutions. 3. a) secondary; 5-ethyl-4-iodo-3methyl-octane. b) primary; 1-bromo-2,3,4-trimethyl-pentane The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. x-axis is the reaction coordinate and Y-axis is energy (enthalpy). (20 points) (CH3)3COH +HCI (CH3)3CCI H2O (CH3)3COH2 () Is the overall reaction endothermic or exothermic?
The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. The S N 2 mechanism. There are two mechanistic models for how an alkyl halide can undergo nucleophilic substitution. In the first picture, the reaction takes place in a single step, and bond-forming and bond-breaking occur simultaneously. (In all figures in this section, 'X' indicates a halogen substituent). This is called an ' SN2' mechanism. The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. (CH3)3C (CH3) COH HCI (CH3)3 CCI H20 (CH3) COH Reaction progress O exothermic is the overall reaction exothermic or endothermic? O endothermic ; Question: The following energy diagram depicts a reaction where an alcohol is converted to an alkyl ... rxn of an alkyl halide w/ ammonia leads to primary, secondary, tertiary amines, and even quaternary ammonium ions. In contrast, the Gabriel synthesis and the rxn of an alkyl halide w/ azide ion form only primary amines. This lab experiment proposes the synthesis of an alkyl halide by reacting the corresponding alcohol with a hydrogen halide in an easy and inexpensive SN1 reaction. 1,2 tert-Butanol reacts readily with HCl and forms the corresponding tert-butyl chloride at room temperature. SN1 mechanisms are unimolecular because its slow step is unimolecular ...
Reactions of Substituent Groups. 1. Oxidation of Alkyl Side-Chains. The benzylic hydrogens of alkyl substituents on a benzene ring are activated toward free radical attack, as noted earlier. Furthermore, S N 1, S N 2 and E1 reactions of benzylic halides, show enhanced reactivity, due to the adjacent aromatic ring. The Grignard reaction is an organic reaction used to produce a variety of products through the reaction of an organomagnesium compound, also known as an electrophilic "Grignard reagent," followed by an acidic reaction. The Grignard reagent is formed by the reaction of an alkyl or aryl halide with magnesium metal via a radical mechanism. Reaction Coordinate Diagram of an S N 2 Reaction. The reaction of hydroxide ion with chloromethane occurs in a single step. The activation energy, E a, reflects the stability of the transition state, which depends upon the structure of the substrate, the nucleophile, and the leaving group. In the reaction of 2-bromobutane in a solution of sodium ethoxide and ethanol, the products will be 2-butene and 1-butene in a ratio of approximately 7:3. Essentially the opposite of Markovnikoff addition, it allows for the prediction of chemical products upon the elimination reaction of an alkyl halide.
a. the alkyl halide is impure b. hydrolysis, a competing reaction, is also taking place c. the initially formed product rearranges d. the ethanol used as a solvent contains a small percentage of water e. the alkyl halide contains three different types of hydrogens that can be attacked by the base Thiols are usually prepared by using the hydrosulfide anion (-SH) as a neucleophile in an S N 2 reaction with alkyl halides. On problem with this reaction is that the thiol product can undergo a second S N 2 reaction with an additional alkyl halide to produce a sulfide side product. This problem can be solved by using thiourea, (NH 2) 2 C=S, as ... The replacement of only one hydrogen atom gives an alkyl halide (or haloalkane). The common names of alkyl halides consist of two parts: the name of the alkyl group plus the stem of the name of the halogen, with the ending -ide. The IUPAC system uses the name of the parent alkane with a prefix indicating the halogen substituents, preceded by ... We provide solutions to students. Please Use Our Service If You’re: Wishing for a unique insight into a subject matter for your subsequent individual research;
Another class of organic molecules contains a carbon atom connected to an oxygen atom by a double bond, commonly called a carbonyl group. The trigonal planar carbon in the carbonyl group can attach to two other substituents leading to several subfamilies (aldehydes, ketones, carboxylic acids and esters) described in this section.
The following reactions are all examples of decarboxylation (loss of CO 2). In the first, bromine replaces the carboxyl group, so both the carboxyl carbon atom and the remaining organic moiety are oxidized. Silver salts have also been used to initiate this transformation, which is known as the Hunsdiecker reaction. The second reaction is an ...
In the presence of a strong acid, an alcohol can be dehydrated to form an alkene. The acid used in this experiment is 85% phosphoric acid and the alcohol is cyclohexanol. The phosphoric acid is a catalyst and as such increases the rate of reaction but does not affect the overall stoichiometry. It can be seen from the balanced reaction
New Window. The Henry's Law constant for 1-bromobutane is estimated as 8.7X10-3 atm-cu m/mole (SRC) derived from its vapor pressure, 42.0 mm Hg (1), and water solubility, 8.69X10+2 mg/L (2). This Henry's Law constant indicates that 1-bromobutane is expected to volatilize from water surfaces (3).
One key message you want to remember is to NEVER do an S N 2 on a tertiary alkyl halide. The carbon is simply too sterically hindered (crowded) and cannot be attacked by the nucleophile. The Nucleophile in S N 2 reactions. At some point in your organic 1 class you will need to determine if the reaction goes by S N 1 or S N 2 reaction.
Both reactions will involve the initial protonation of the alcohol to form an oxonium ion. Then, the loss of the water leaving group will yield a tertiary carbocation. Where the reactions differ is that the chloride anion is a good nucleophile, whereas the conjugate base of sulfuric acid is essentially non-nucleophilic.
The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. Match the reagents/intermediates/products below with the appropriate labels (A, B, C, or D) in the diagram (Drag the letter to the appropriate structure(s)).
Alkyl halide or haloalkanes are formed by the replacement of hydrogen atoms in an aliphatic hydrocarbon by halogen atoms (Fluorine, chlorine, bromine or iodine). They can also be manufactured from any organic precursors such as alkanes, alkenes, or alcohols and carboxylic acids. Generally, alkyl halides contain hydrogen atoms attached to the sp ...
The hydroxyl group of an alcohol is replaced by halogen on reaction with concentrated halogen acids, phosphorus halides or thionyl chloride. Thionyl chloride is preferred because in this reaction alkyl halide is formed along with gases SO 2 and HCl. The two gaseous products are escapable, hence, the reaction gives pure alkyl halides.
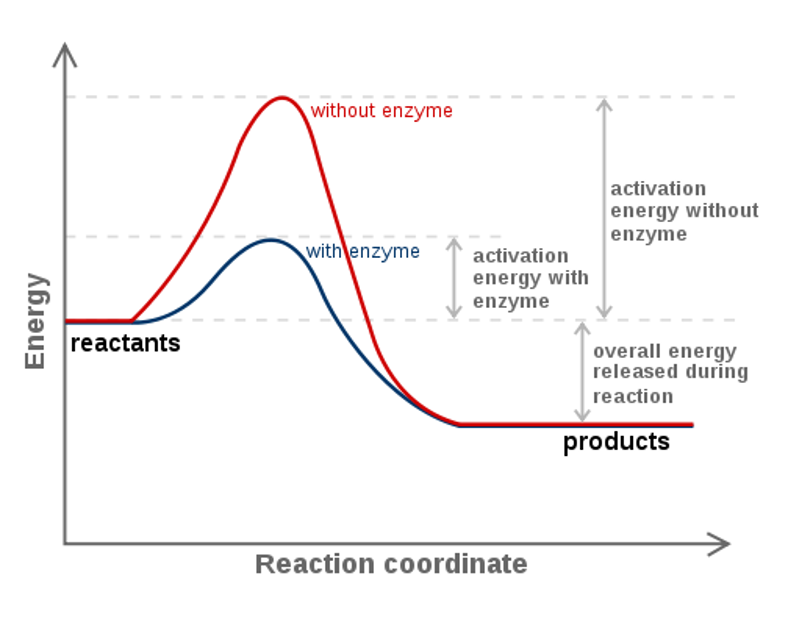
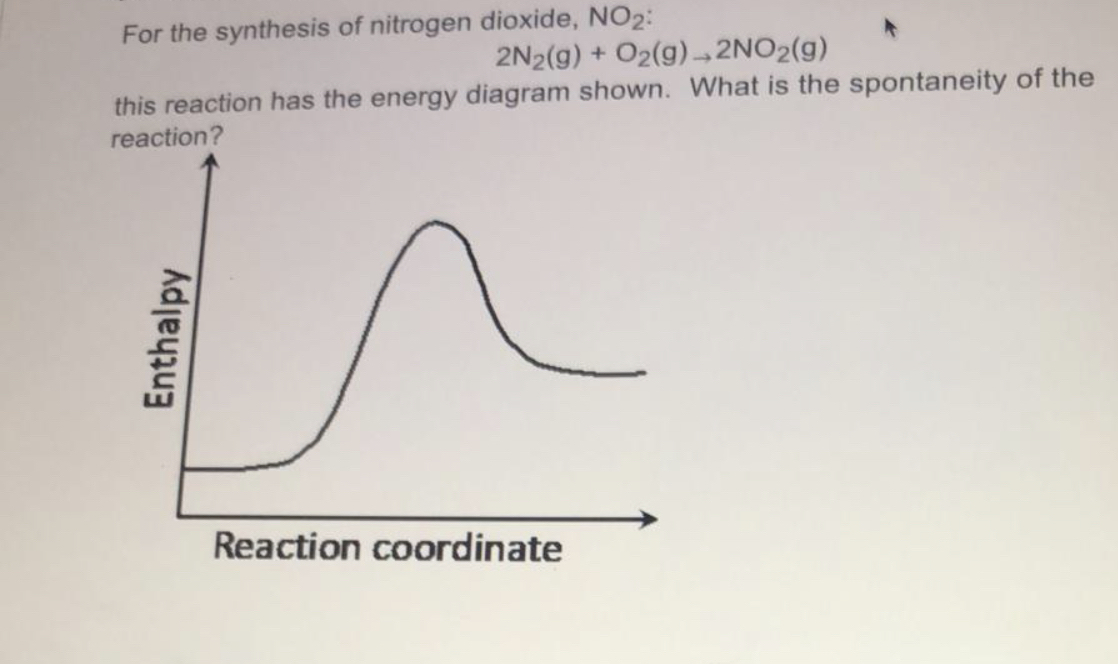
Figure 1.18 The Change in the Gibbs free energy (ΔG) is the Driving Force of the Reaction. In this diagram, equilibrium (where ΔG = 0) is represented by the black line of the horizontal axis. Using Le Chatlier’s Principle, it is possible to predict the behavior of a reaction when perturbations are made that shift the reaction out of ...
Complete the Following Reactions: Hydrolysis of Esters. The reverse reaction of ester formation can be used to breakdown esters into a carboxylic acid and an alcohol. This reactions requires the incorporation of water into the ester linkage, and is thus called a hydrolysis reaction.
100k Terms - Free ebook download as Text File (.txt), PDF File (.pdf) or read book online for free.
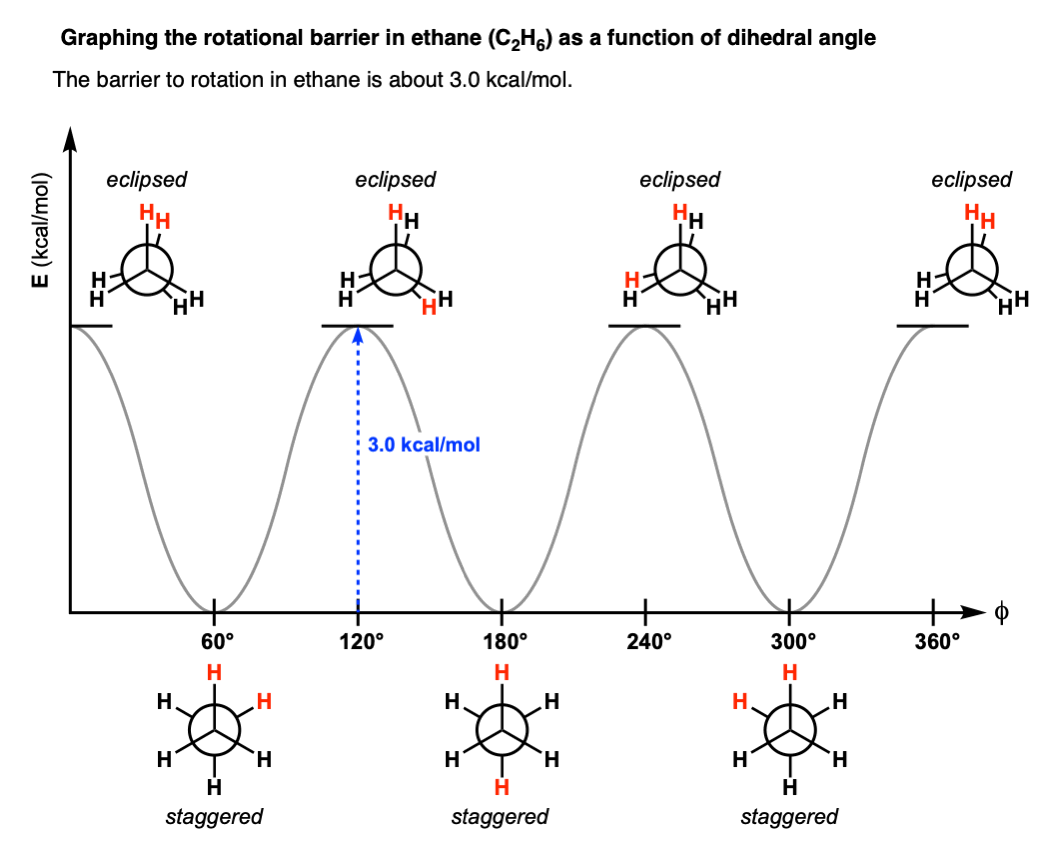
•An energy diagram is a schematic representation of the energy changes that take place as reactants are converted to products. •An energy diagram plots the energy on the y axis versus the progress of reaction, often labeled as the reaction coordinate, on the x axis. •The energy difference between reactants and products is H°.
The Wittig Reaction. The most important use of ylides in synthesis comes from their reactions with aldehydes and ketones, which are initiated in every case by a covalent bonding of the nucleophilic alpha-carbon to the electrophilic carbonyl carbon. Ylides react to give substituted alkenes in a transformation called the Wittig reaction.
The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. x-axis is the reaction coordinate and Y-axis is energy (enthalpy). (20 points) (CH3)3COH +HCI (CH3)3CCI H2O (CH3)3COH2 () Is the overall reaction endothermic or exothermic?
5. Arrange the following alkyl halides in order of decreasing boiling point. 6. Predict the solvent with great alkyl halide solubility. a) water or hexane. b) water or 1-octanol. c) water or benzene. d) water or acetone. Solutions. 3. a) secondary; 5-ethyl-4-iodo-3methyl-octane. b) primary; 1-bromo-2,3,4-trimethyl-pentane
Acid catalyzed dehydration of secondary / tertiary alcohols. We'll take a look at a mechanism involving solvolysis during an E1 reaction of Propanol in Sulfuric Acid.. Step 1: The OH group on the pentanol is hydrated by H 2 SO 4.This allows the OH to become an H 2 O, which is a better leaving group.; Step 2: Once the OH has been hydrated, the H 2 O molecule leaves, taking its electrons with it.
















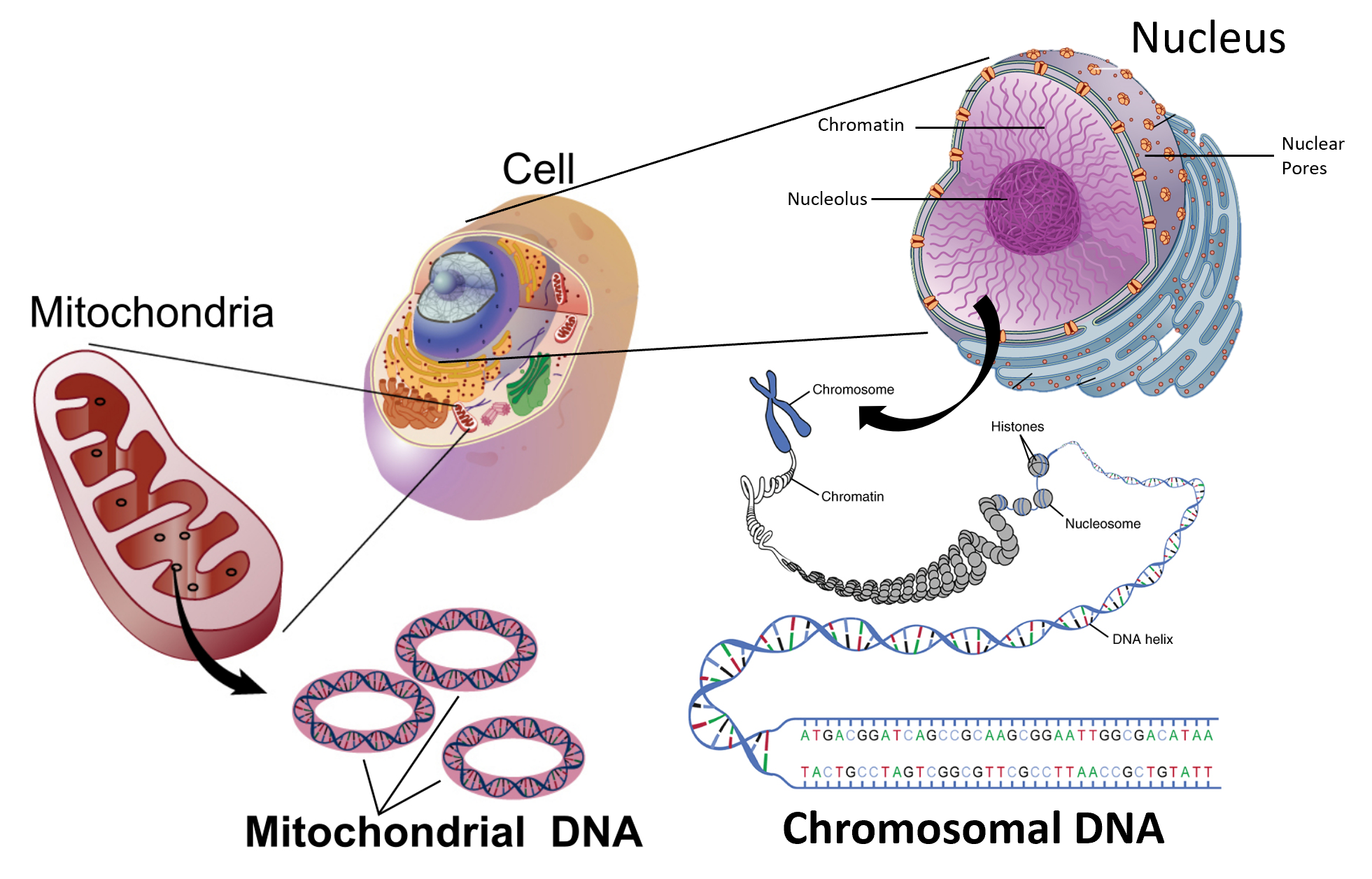
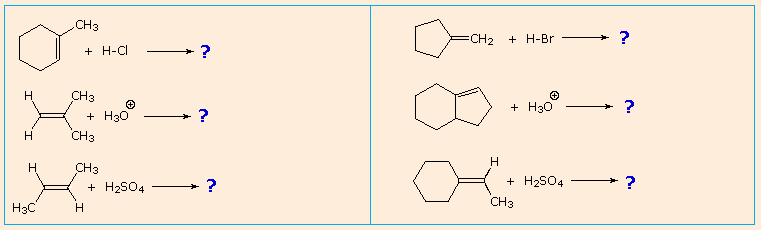




0 Response to "44 the following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide"
Post a Comment