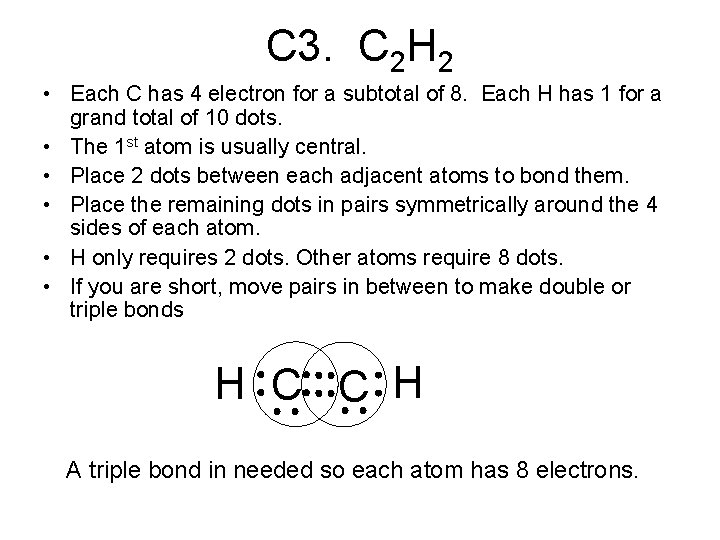
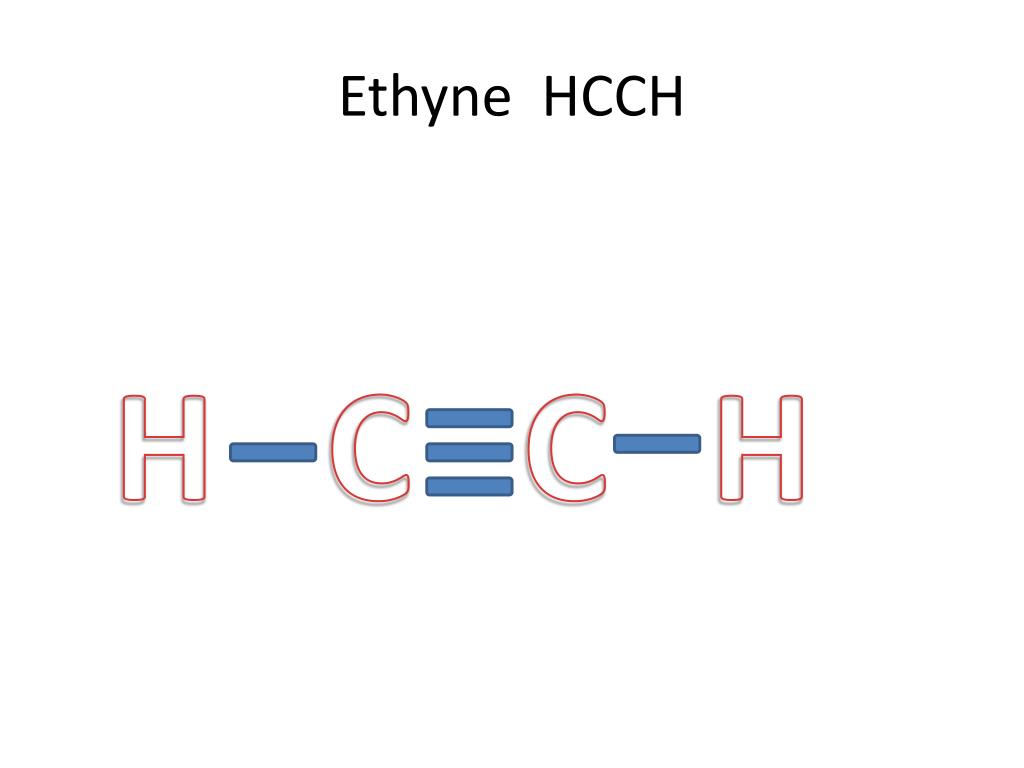
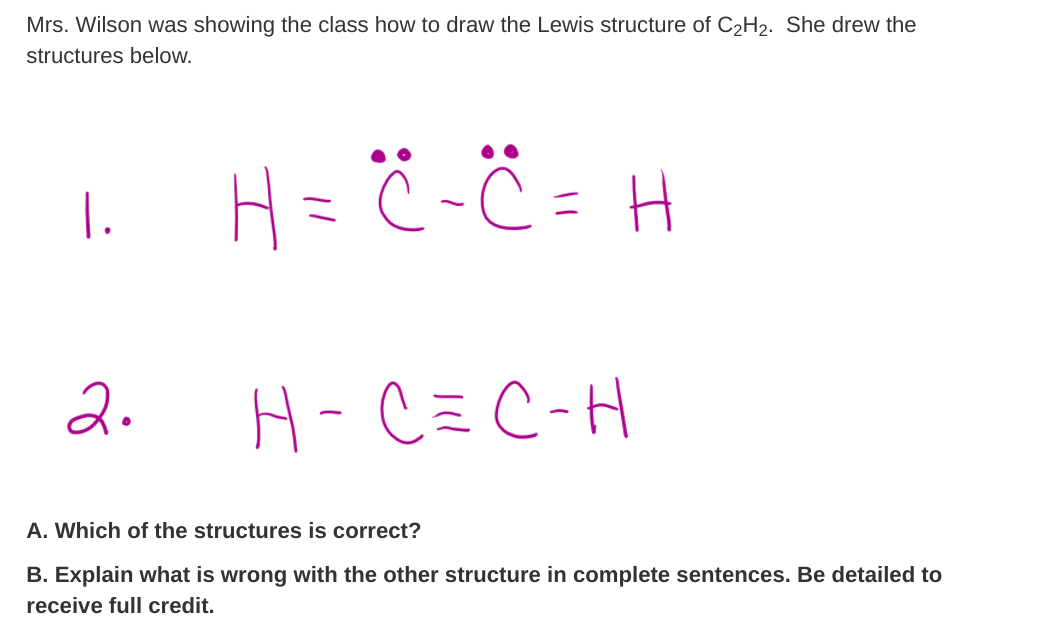
45 lewis diagram for c2h2
Sf4 2 lewis structure [email protected] masc. proper name, Anglo-French form of French Louis (see Louis).
"worthless," 1711, from adjectival phrase (see good (adj.)).
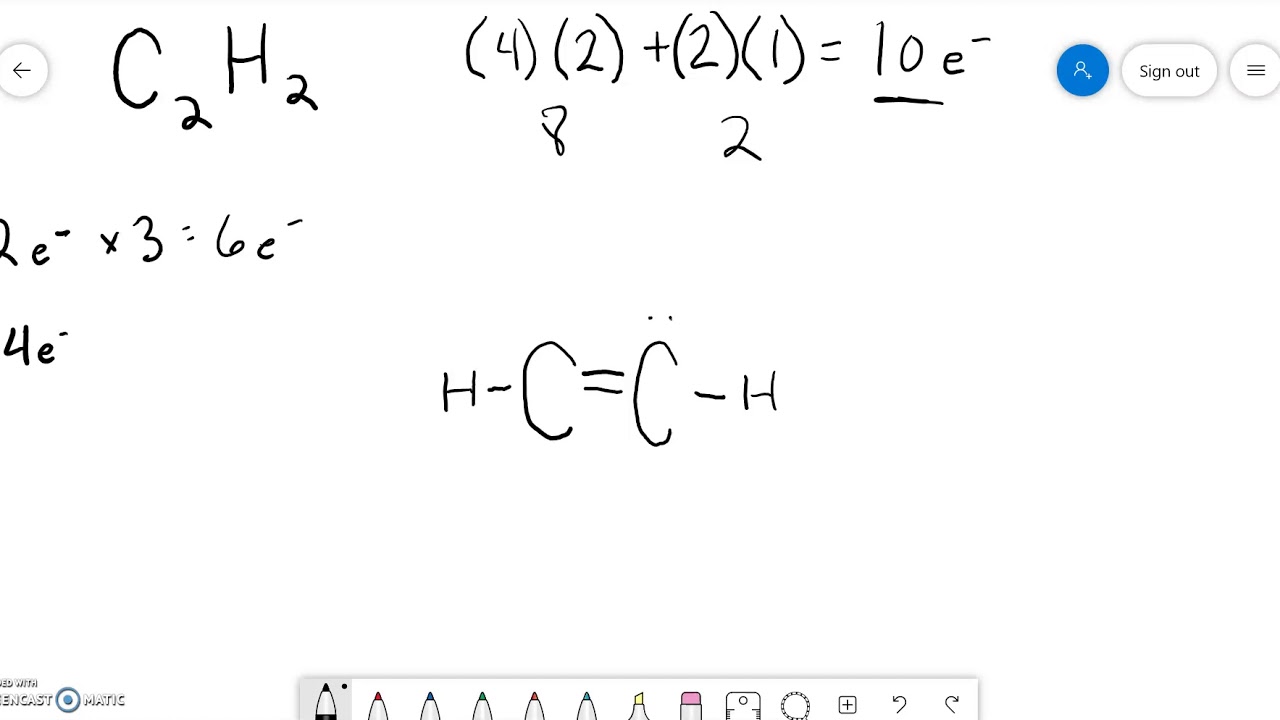
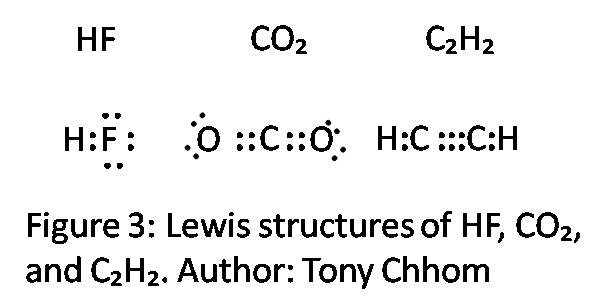
Lewis diagram for c2h2
C2h2 Acetylene Ethyne Lewis Structure . C2h2 Ve C2h6 Nin Lewis Gosterimi Acil Yapabilir Misiniz Eodev Com . How To Determine The Lewis Dot Structure For C2h2cl2 Quora . Http Www Youtube Com Watch V Qojoaosk5ui Lewis Chemistry Math Equations . Location: Share : Newer Posts Older Posts prefix usually meaning "away, opposite, completely," from Old English for-, indicating loss or destruction, but in other cases completion, and used as well with intensive or pejorative force, from Proto-Germanic *fur "before, in" (source also of Old Norse for-, Swedish för-, Dutch ver-, Old High German fir-, German ver-); from PIE *pr-, from root *per- (1) "forward," hence "in front of, before, toward, near, against." In verbs the prefix denotes (a) intensive or completive action or process, or (b) action that miscarries, turns out for the worse, results in failure, or produces adverse or opposite results. In many verbs the prefix exhibits both meanings, and the verbs frequently have secondary and figurative meanings or are synonymous with the simplex. [Middle English Compendium] Probably originally in Germanic with a sense of "forward, forth," but it spun out complex sense developments in the historical languages. Disused as a word-forming element in Modern English. Ultimately from the same root as fore (adv There are no lone pairs on carbon or hydrogen atoms. In this tutorial, we are going to learn how to draw lewis structure of C2H2.
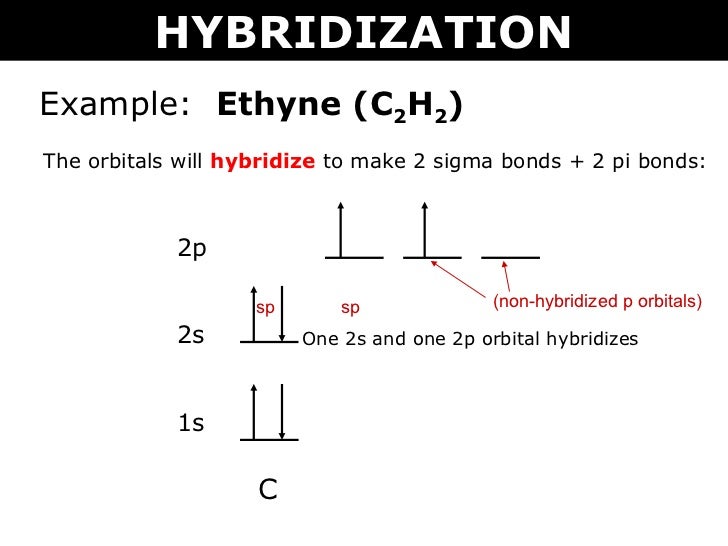
Lewis diagram for c2h2. Lewis dot structure is a diagram that represents the number of valence electrons of an element through dots around the element symbol. Learn about Lewis dots and understand single bonds, double ... The lewis structure of c2h2 has 1 triple bond. For the HCCH Lewis structure youll need to form a triple bond between the two carbon atoms. Triple bonds are always shorter than single bonds. There are no lone pairs on carbon or hydrogen atoms. For each C one can explain the bonds through sp hybridization a triple bond and one single bond. Learn to determine if C2H2 is polar or nonpolar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structure and look an. In C2H6 there is quite a little difference in electronegativities between Hydrogen and Carbon atoms which means that the C-H bonds are nonpolar. 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, ...Oct 3, 2010 · Uploaded by kentchemistry.com
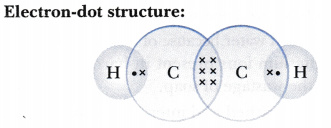
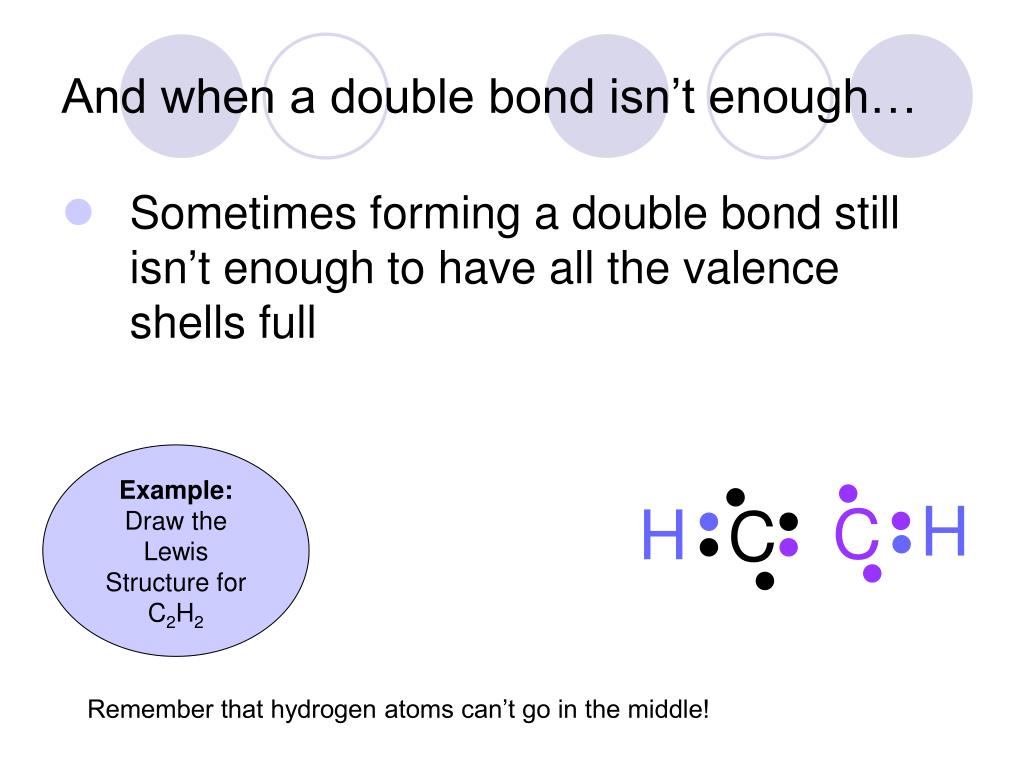
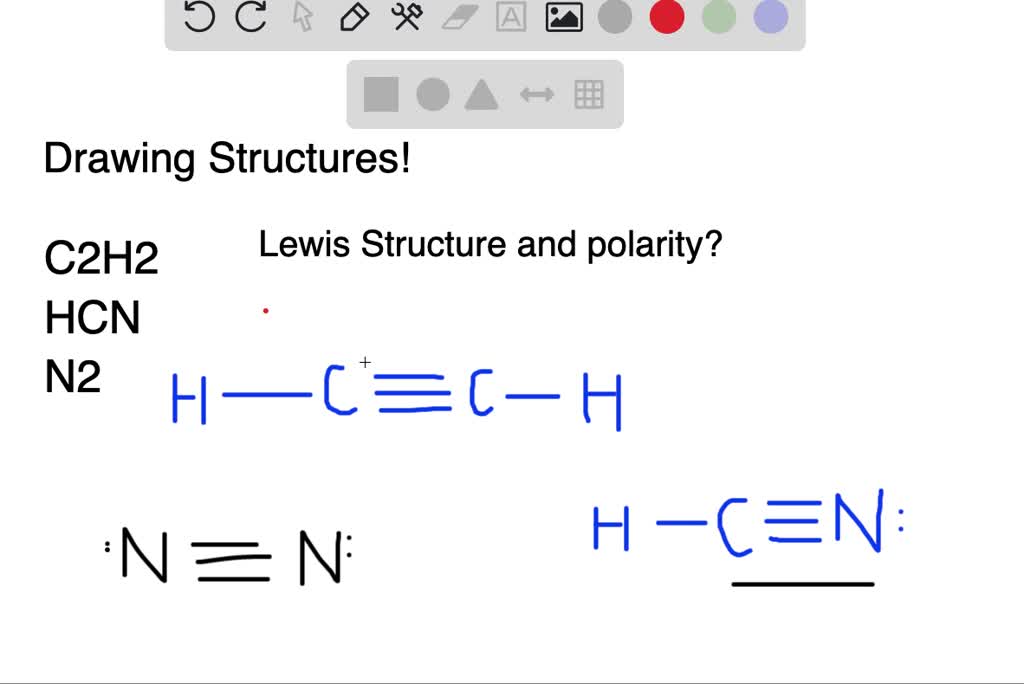
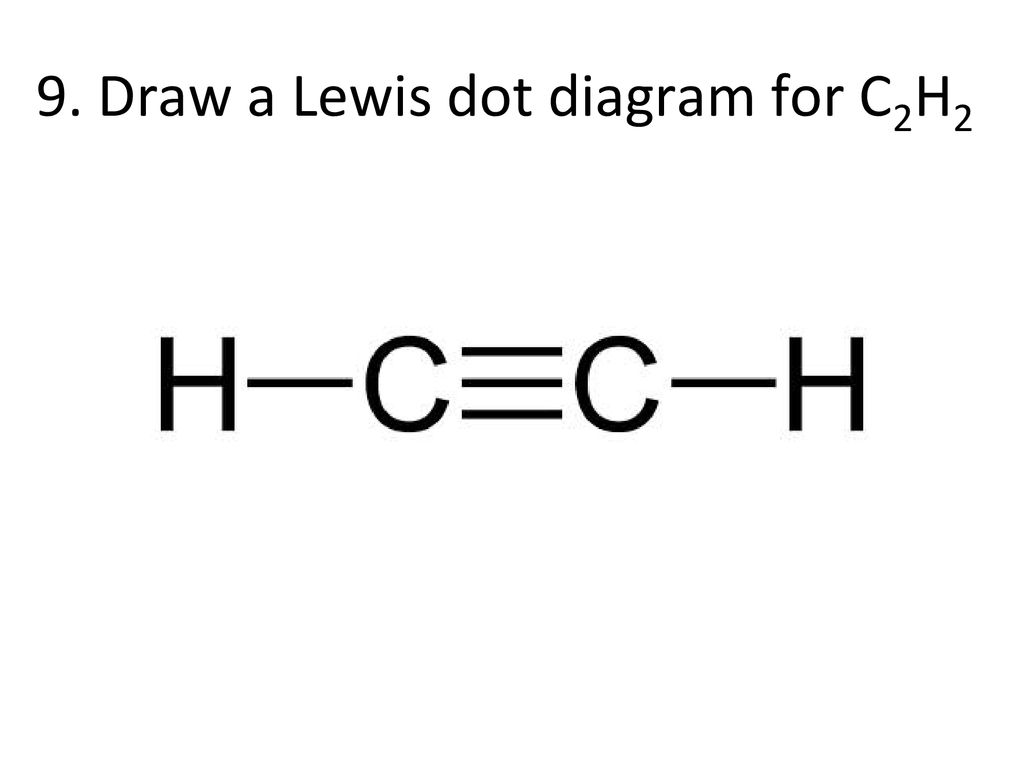
1918 (Venn's diagram is from 1904), named for English logician John Venn (1834-1923) of Cambridge, who explained them in the book "Symbolic Logic" (1881). What is the Lewis structure of C2H4? For C2H4. In C2H4, if we look into the lewis structure, we will see that there are three bonded pairs of electrons around each carbon and zero lone pair. According to the VSEPR chart, the shape of the ethene molecule is trigonal planar. There are two triangles overlapping each other as we can see in the diagram. In drawing the Lewis structure for C2H2 (also called ethyne) you'll find that you don't have enough valence electrons available to satisfy the octet for each element (if you use only single bonds). The solution is to share three pairs of valence electrons and form a triple bond between the Carbon atoms in C2H2 . Diagrama Lewis C2h2 Estructura de Lewis del Acetileno C2H2 Estructura de Lewis del Acetileno C2H2 Estructura de Lewis Wikipedia la enciclopedia libre HCCH Lewis Structure. H CC Hacetileno El acetileno tiene una estructura lineal que se explica admitiendo una hidridación sp en cada uno de los átomos de carbono.
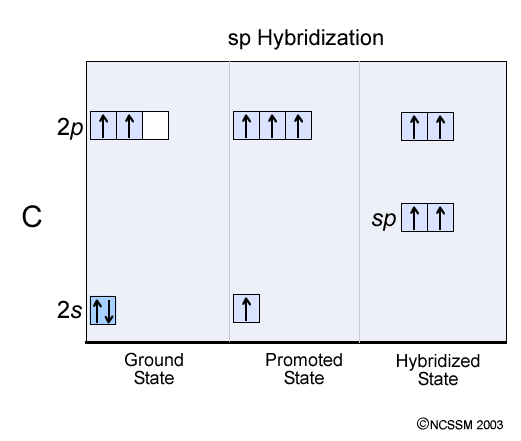
The Lewis structure of an element is a representation of a chemical symbol surrounded by dots which represent the valence of electrons. Step 1: Calculation of total number of valence electrons for molecule to draw the Lewis structure. Total number of valence electrons in C2H2 molecule = 2 x 1 + 4 + 6 = 12 e step 2: Distribution of electrons ... PCl3 lewis structure In this lewis structure of PCl3, center phosphorus atom has made three single bonds with three chlorine atoms. There is a lone pair on center phosphorus atom and each chlorine atom also has three lone pairs. ... In the case of C2H2 we have SP hybridization, as 2 SP hybridized orbitals (one for each carbon atom) are ... 1610s, "an illustrative figure giving only the outlines or general scheme of the object;" 1640s in geometry, "a drawing for the purpose of demonstrating the properties of a figure;" from French diagramme, from Latin diagramma "a scale, a musical scale," from Greek diagramma "geometric figure, that which is marked out by lines," from diagraphein "mark out by lines, delineate," from dia "across, through" (see dia-) + graphein "write, mark, draw" (see -graphy). Related: Diagrammatic; diagrammatically. The verb, "to draw or put in the form of a diagram," is by 1822, from the noun. Related: Diagrammed; diagramming. Oct 21, 2017 — Note, hydrogen atoms (h) should not have lone pair. C2h2 Lewis Structure Tutorial How To Draw The For Ethyne Or Acetylene
) Quiz your students on Lewis Dot Diagram Structure For IF3, Bond Angle, Hybridization, Polar or Nonpolar using our fun classroom quiz game Quizalize and personalize your teaching. E(I) Number of I-F bonds = N. In the best Lewis structure the formal charge of the onygrn is B. Viewed after searching for: lewis dot structure for covalent compounds.
Acetylene | C2H2 | CID 6326 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards ...
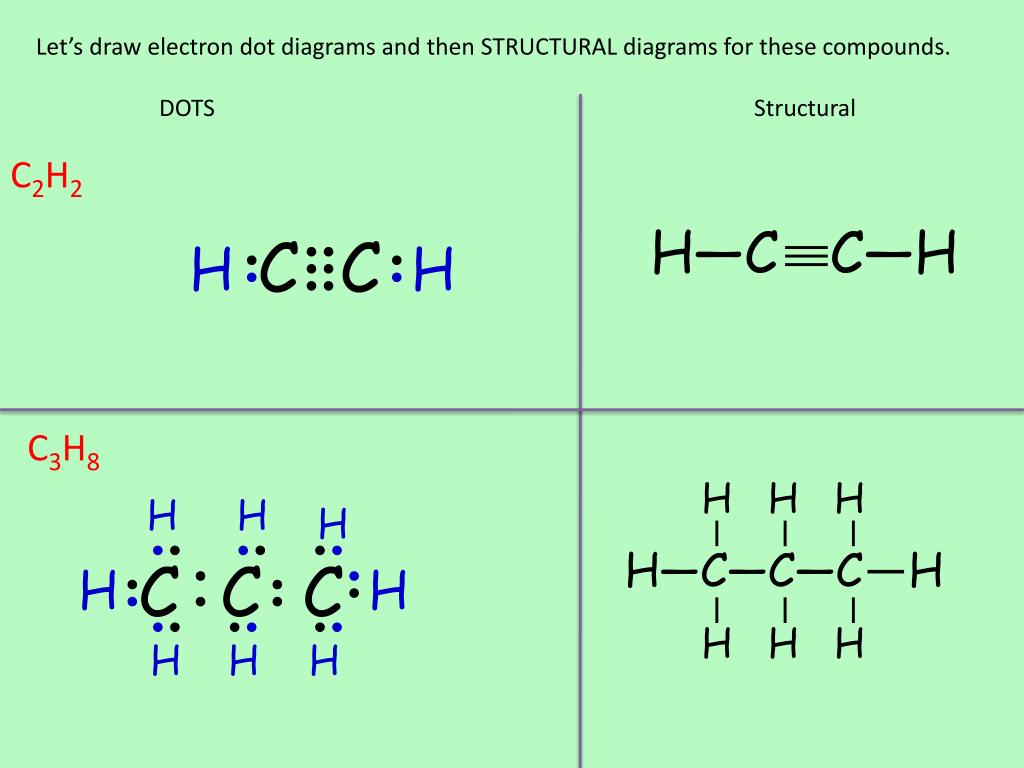
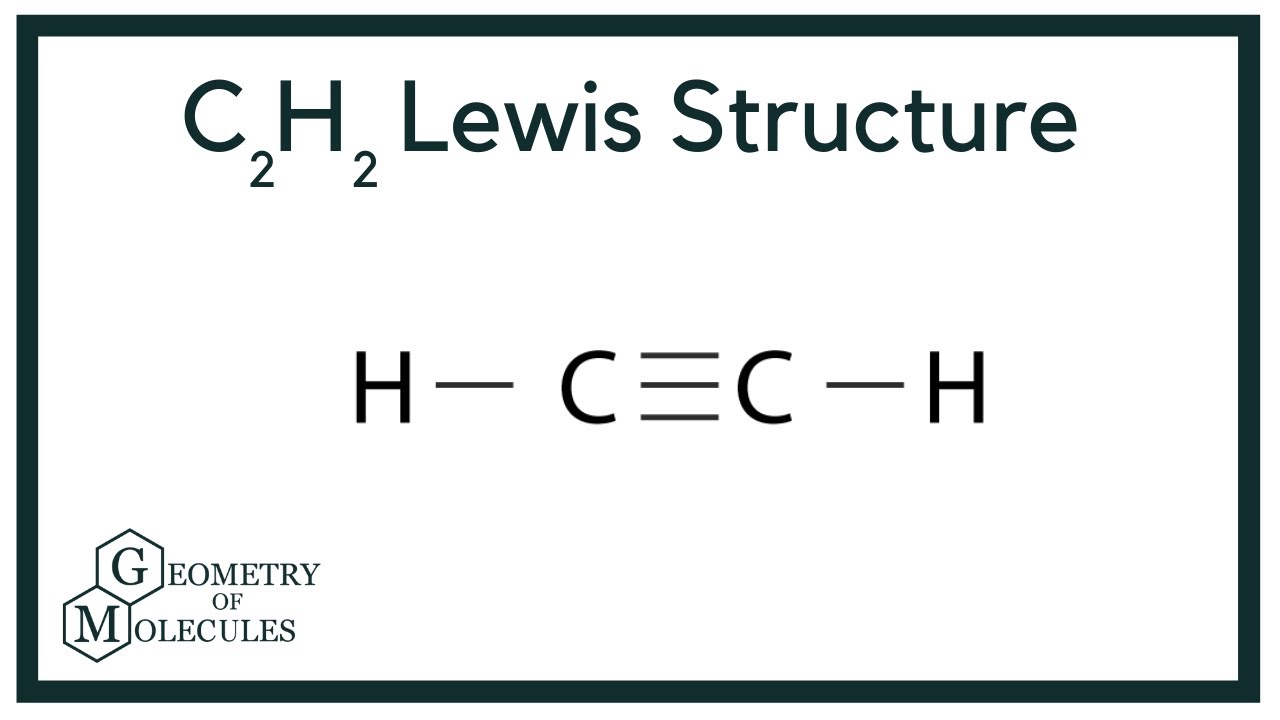
Lewis structure for c2h2. Since each line represents 2 shared electrons the carbon atoms share 8 electrons. Total valence electron Group Valence Electrons. In drawing the Lewis structure for C 2 H 2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds.
The Lewis Structure for c2h2 shows that there are two carbon atoms each with 3 single bonds to hydrogen. Each carbon has 2 lone pairs which form the double bond between the carbons. This molecule is linear because it only has one C-C bond, if it were bent then it would be an orbital hybrid.
PCl5 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram, BCl3 Lewis Structure, Molecular Geometry, and Hybridization, PH3 Lewis Structure, Molecular Geometry, and Hybridization. Ethyne, which has the formula C2H2, is a nonpolar molecule. The C2H2 molecule is made of two sigma-bonds and two pi-bonds, with a bond angle of 180 degrees.
C2H4 Lewis Structure Ethylene Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure of C2H4 molecule. Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond.
"mass brawl" (one in which all may participate), 1918, from earlier adjective use (1868), especially in reference to open horse races, American English. Earlier as a noun in reference to free-for-all horse and motorcar races.
C2H6 Lewis Structure. Lewis structure is a 2D representation of the compound, which represents only the valance shell electrons of the atoms in the molecule. It is based on the octet rule i.e. every atom tends to complete its octet ( 8 electrons) either by gaining or losing electrons except Hydrogen and Helium as they complete their duplet.
1550s, "be taken or regarded as," also "be in favor of," from go (v.) + for (adv.). Meaning "attack, assail" is from 1880. Go for broke is from 1951, American English colloquial.
Lewis Structure For C2h2 C2H2 Lewis Structure Tutorial How to Draw the Lewis Lewis Dot Structure of C2H2 or CHCH Acetylene or ethyne Organic chemistry. The solution is to share three pairs of valence electrons and form a triple bond between the Carbon atoms in C 2 H 2. Final Exam Review - Chemistry with Coccine at.
15 Schematic Diagram Example. The arcgis schematics extensionworks with arcgis desktop and arcgis server to enable generating, visualizing, and manipulating schematic diagrams from network data coming from a. The schematic diagram in figure 1 completely describes the process of power generation in thermal (or steam). Gainclone amplifier circuit,stereo 40W using…
1 answerThe number of valence electrons in carbon and hydrogen is 4 and 1 respectively. We denote electrons as a dot in Lewis structure. The total number of valence ...
Lewis Structures for C2H2. Step-by-step tutorial for drawing the Lewis Structure for C2H2.May 15, 2013 · Uploaded by Wayne Breslyn
SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots( ) but a lone pair of two electrons is shown by dots[ ].
In drawing the Lewis structure for C 2 H 2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds. A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure Ethyne or AcetyleneFor the C2H2 structure use the periodic table to find the total.
Nov 24, 2021 · CH3OCH3 Lewis Structure. Lewis Structure is the initial step towards finding out about the chemical bonding in a given molecule. It deals with the valence or the outermost shell electrons which come together in pairs and form covalent bonds between atomic elements.
14+ C2H2 Lewis Structure. Which set of bonds is arranged in order of increasing polarity? (a) ph3 (b) ascl3 (c) sih4 (d) sbcl3 (e) h2s. Use lewis structures to show this sharing. Please help me with this lewis structure: Source: study.com. C2h2 would turn into what is known as ethyne. Source: i.ytimg.com.
Is C2H2 linear or bent? C2H2 has a linear shape given its molecular geometry is linear and all the atoms are arranged symmetrically. To summarize this article on C2H2 Lewis structure, we can say that, There are ten valence electrons for Ethyne.
What is the Lewis structure of C2H2? C2H2 (Acetylene | Ethyne) Lewis Structure. C2H2 (acetylene or ethyne) contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds. There are no lone pairs on carbon or hydrogen atoms. What does C2H2 look like?
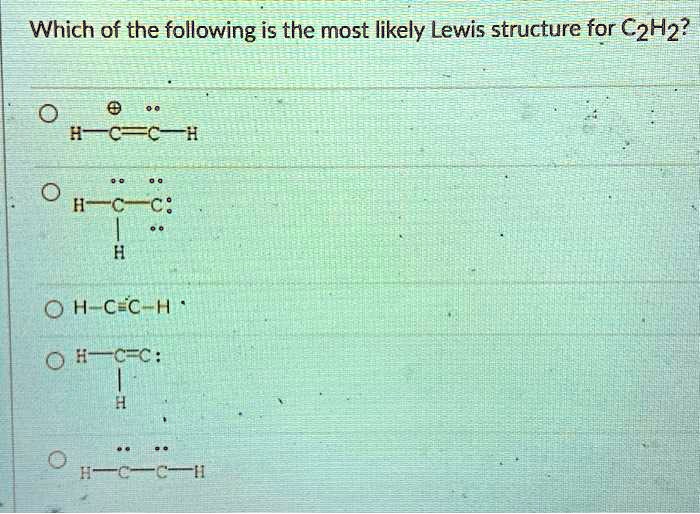
Answer to The Lewis structure for acetylene (C2H2) is drawn as Expl....
In the Lewis structure of C2H2, Carbon atoms take the central position, and the Hydrogen atoms are arranged around it. Both Carbon atoms share one valence electron with one Hydrogen atom by forming a single bond. And to complete their octets, both these atoms share their remaining three valence electrons in the outer shell by forming a triple ...
Nov 30, 2021 · C2H2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram Acetylene or C2H2 is the simplest alkyne and a hydrocarbon that is colorless and has a garlic-like odor. It is highly reactive to atmospheric temperature and lacks oxygen being an unsaturated compound due to the presence of two carbon atoms bonded with a triple bond.
Drawing the Lewis Structure for C2H2 (Ethyne or Acetylene) For C2H2 you might have a complete of 10 valence electrons to work with. In drawing the Lewis structure for C2H2 (additionally known as ethyne) you will discover that you do not have sufficient valence electrons out there to fulfill the octet for every component (should you use solely ...
1550s, possibly an alteration of tip for tap "blow for blow," from tip (v.3) "tap" + tap "touch lightly." Perhaps influenced by tit (n.2).
A Lewis structure also helps to make a prediction about the geometry of a molecule. Misc hca chemistry customizable and printable lewis dot diagram worksheet 50 structure practice in 2020 (with images electron calculator free photos ppt drawing structures a tutorial on writing Lewis Structures of Monatomic Ions.
Old English for "before, in the sight of, in the presence of; as far as; during, before; on account of, for the sake of; in place of, instead of," from Proto-Germanic *fur "before; in" (source also of Old Saxon furi "before," Old Frisian for, Middle Dutch vore, Dutch voor "for, before;" German für "for;" Danish for "for," før "before;" Gothic faur "for," faura "before"), from PIE root *per- (1) "forward," hence "in front of, before," etc. From late Old English as "in favor of." For and fore differentiated gradually in Middle English. For alone as a conjunction, "because, since, for the reason that; in order that" is from late Old English, probably a shortening of common Old English phrases such as for þon þy "therefore," literally "for the (reason) that."
Lewis Dot Structures 1. 83 ug/L from 1980 to 2000 (3). This corresponds to a Tetrahedral geometrical shape having a bond angle 109. A Lewis structure is a graphic representation of the electron distribution around atoms. Because Chromium has 24 protons/electrons on a Bohr Diagram it will have 24 dots. The Lewis Dot Structure for CH4 is shown above.
Ch4 hybridization diagram [email protected] H: 1s1. If you know one, then you always know the other. Carbon's electron configuration is 1s 2 2s 2 2p 2 in the ground state. Heat exchanger device and method for heat removal or transfer.
"fabulous, dangerous creature," 1871 ("Jabberwocky"), coined by Lewis Carroll.
Identify the hybridization of all interior atoms for the molecule IF5, according to valence bond theory, in the diagram showing orbital overlap below. Draw an appropriate Lewis structure for CH2CHCH3.
Question Give the Lewis dot structure of C2H2. Ethyne: C2H2C2H2 is the chemical formula of the ethyne molecule. The common name of this molecule is acetylene. It is the simplest alkyne (unsaturated hydrocarbon molecules containing at least one triple bond) molecule. Answer n C2H2C2H2, two carbon atoms are present that are bonded to each other by a triple ...
C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond angle. C2H2 is a chemical formula for Ethyne, a gaseous alkyne hydrocarbon. It has been used widely for welding and cutting purposes. This molecule is also known by the name Acetylene. The compound has a simple structure and is made up of two carbon atoms and two hydrogen atoms.
There are no lone pairs on carbon or hydrogen atoms. In this tutorial, we are going to learn how to draw lewis structure of C2H2.
prefix usually meaning "away, opposite, completely," from Old English for-, indicating loss or destruction, but in other cases completion, and used as well with intensive or pejorative force, from Proto-Germanic *fur "before, in" (source also of Old Norse for-, Swedish för-, Dutch ver-, Old High German fir-, German ver-); from PIE *pr-, from root *per- (1) "forward," hence "in front of, before, toward, near, against." In verbs the prefix denotes (a) intensive or completive action or process, or (b) action that miscarries, turns out for the worse, results in failure, or produces adverse or opposite results. In many verbs the prefix exhibits both meanings, and the verbs frequently have secondary and figurative meanings or are synonymous with the simplex. [Middle English Compendium] Probably originally in Germanic with a sense of "forward, forth," but it spun out complex sense developments in the historical languages. Disused as a word-forming element in Modern English. Ultimately from the same root as fore (adv
C2h2 Acetylene Ethyne Lewis Structure . C2h2 Ve C2h6 Nin Lewis Gosterimi Acil Yapabilir Misiniz Eodev Com . How To Determine The Lewis Dot Structure For C2h2cl2 Quora . Http Www Youtube Com Watch V Qojoaosk5ui Lewis Chemistry Math Equations . Location: Share : Newer Posts Older Posts








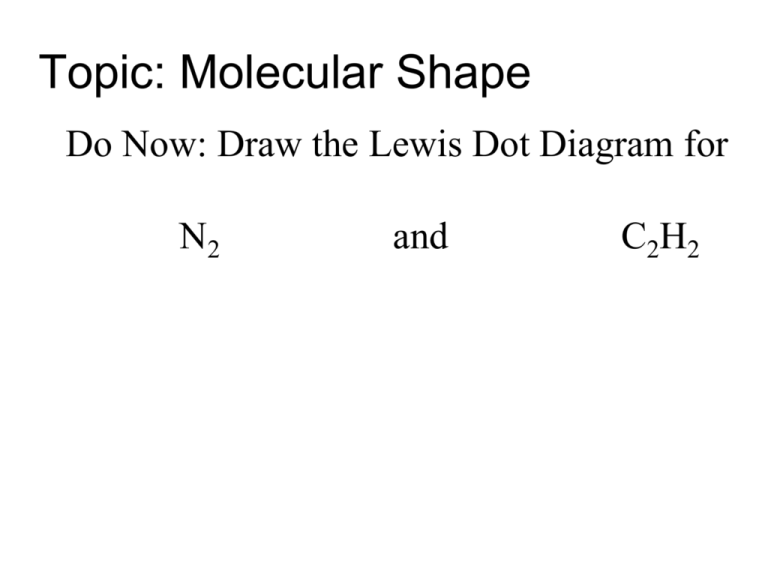














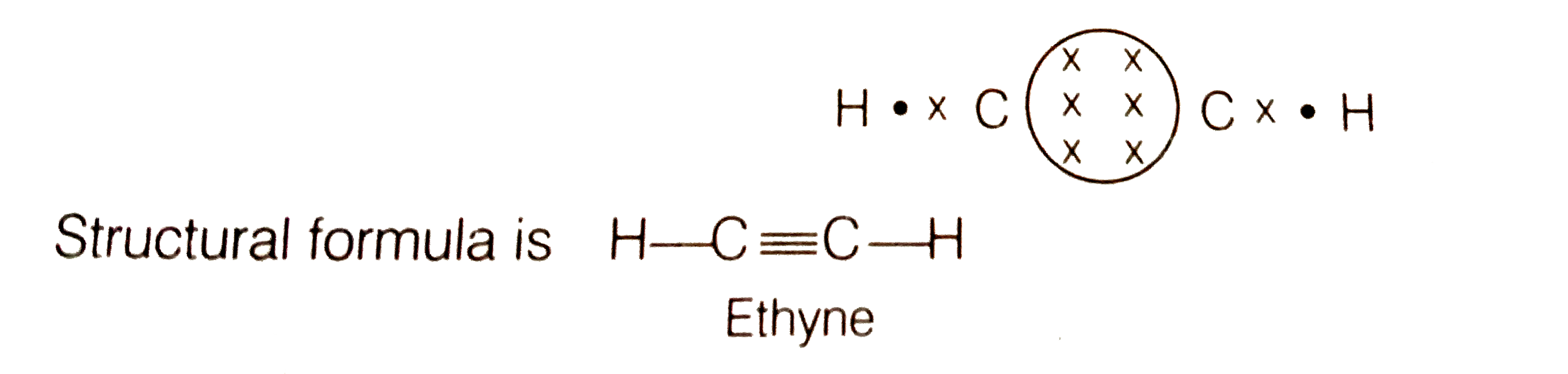
0 Response to "45 lewis diagram for c2h2"
Post a Comment