45 what is the basis for exceptions to the aufbau diagram
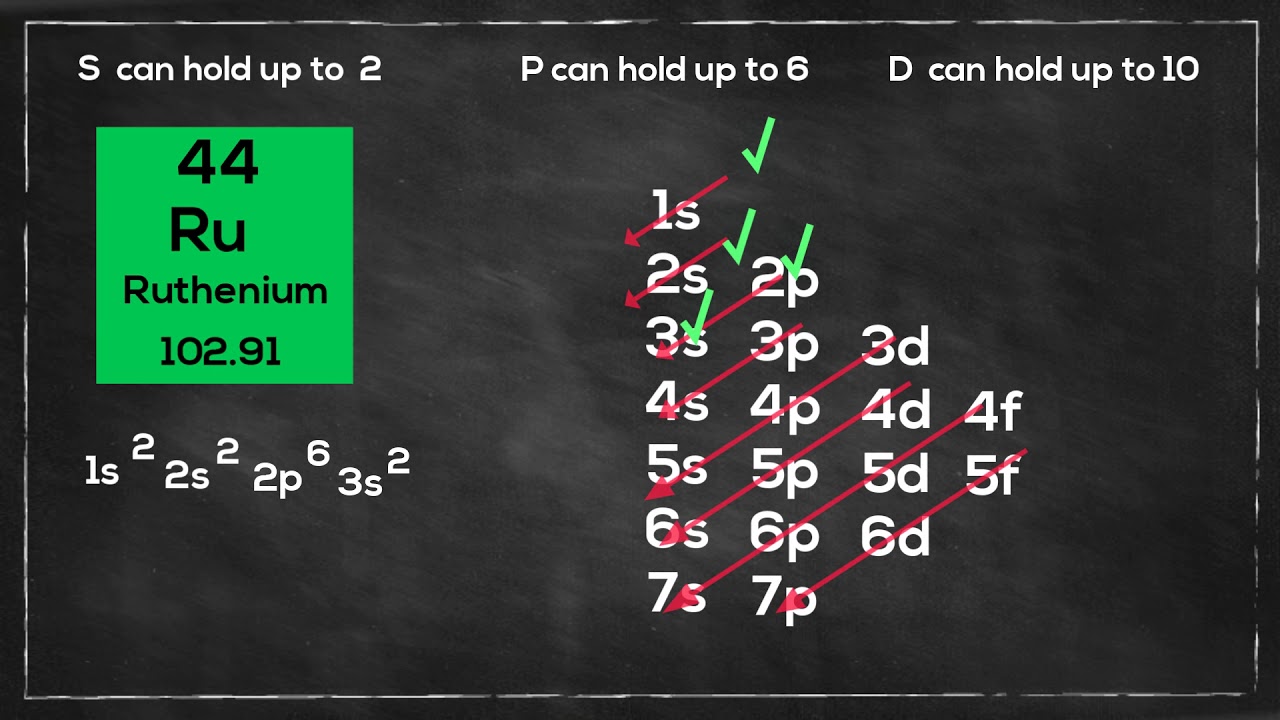
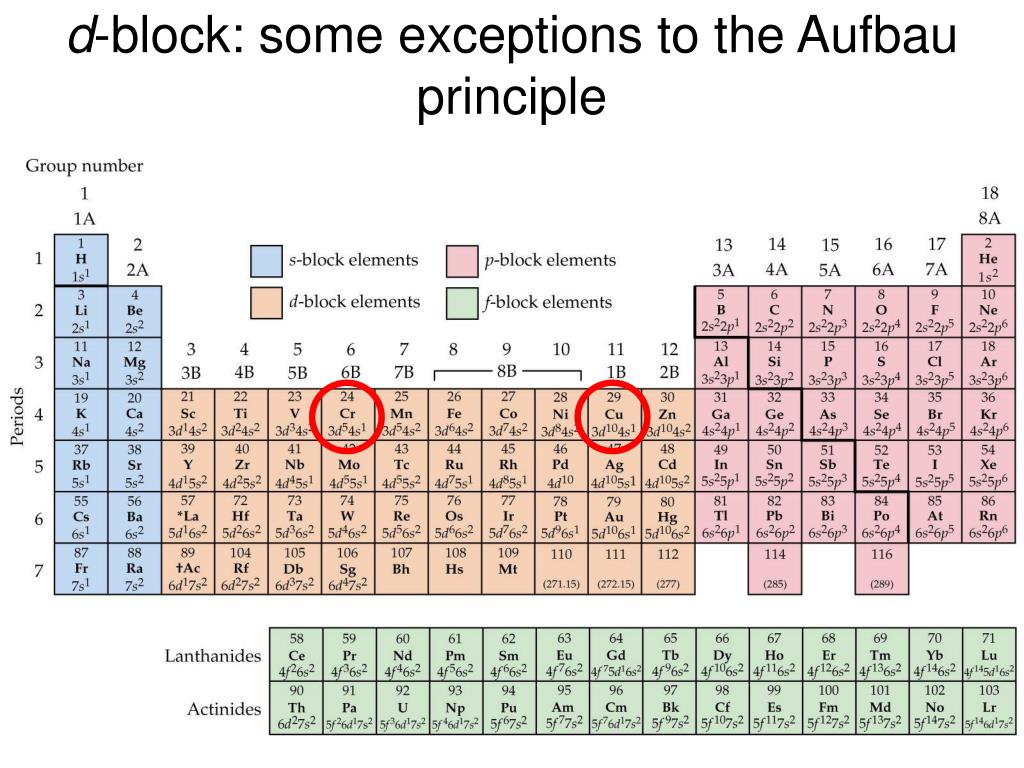
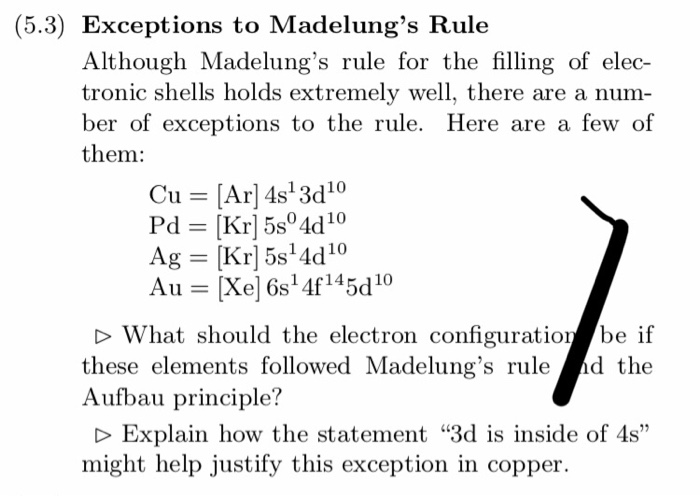
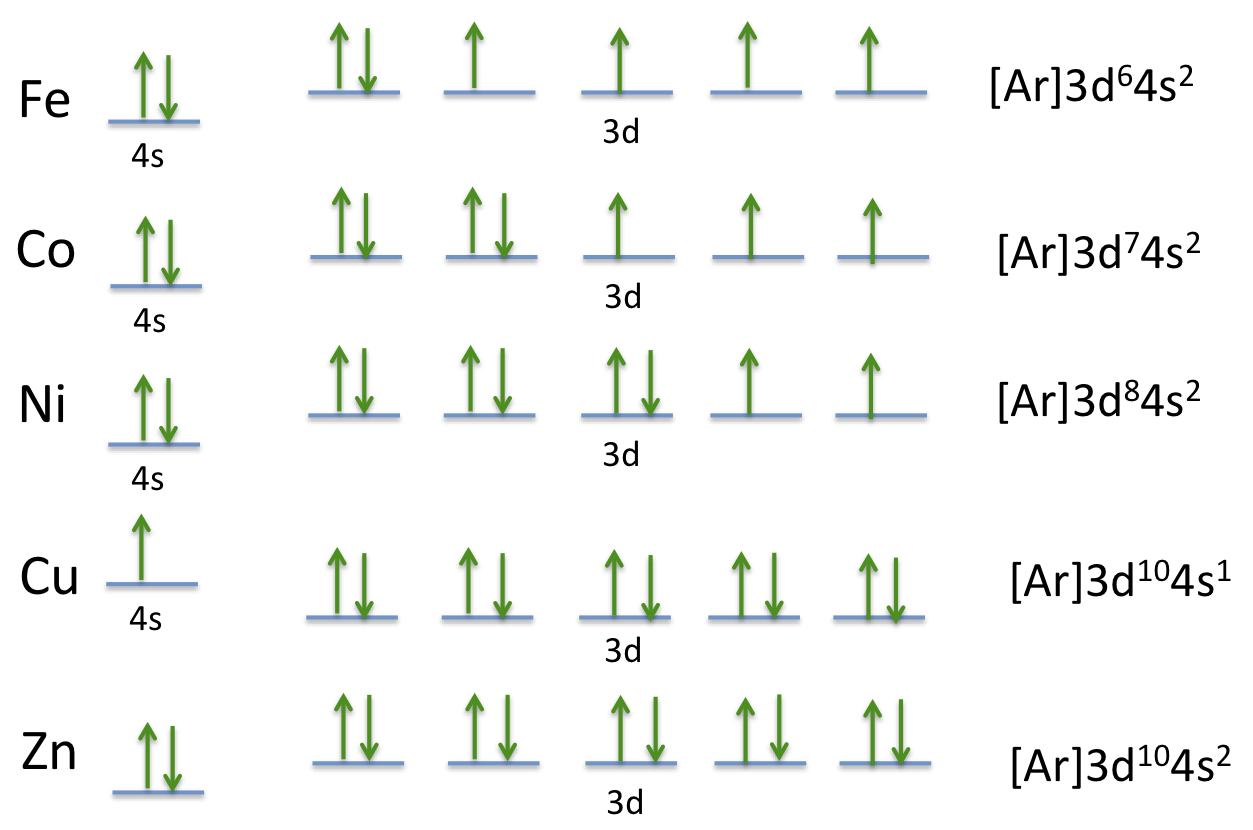
Aufbau Principle Exceptions Like most rules, there are exceptions. Half-filled and completely filled d and f subshells add stability to atoms, so the d and f block elements don't always follow the principle. For example, the predicted Aufbau configuration for Cr is 4s 2 3d 4, but the observed configuration is actually 4s 1 3d 5.
What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels.
The electronic configuration of chromium is [Ar]4s13d5 and not [Ar]4s23d4 (as the Aufbau principle suggests). This exception is attributed due to several ...

What is the basis for exceptions to the aufbau diagram
The Aufbau Principle To determine the electron configuration for any particular atom, we can “build” the structures in the order of atomic numbers. Beginning with hydrogen, and continuing across the periods of the periodic table, we add one proton at a time to the nucleus and one electron to the proper subshell until we have described the electron configurations of all the …
What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels.
Despite the exceptions, the Aufbau principle is useful in chemistry courses where students discover the fundamental rules about the atomic structure and properties of elements. A chart or diagram may be used to show how the principle works for various example elements.
What is the basis for exceptions to the aufbau diagram.
30 May 2018 · 2 answersWith every electron stationed in its own orbital or paired off with each other in the higher energy level, the energy level is balanced and ...
18 Feb 2020 — The aufbau principle states: In the ground state of an atom, atomic orbitals are filled by electrons in the order of their increasing ...
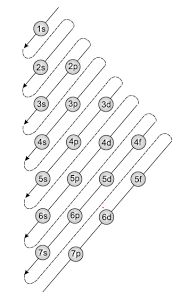
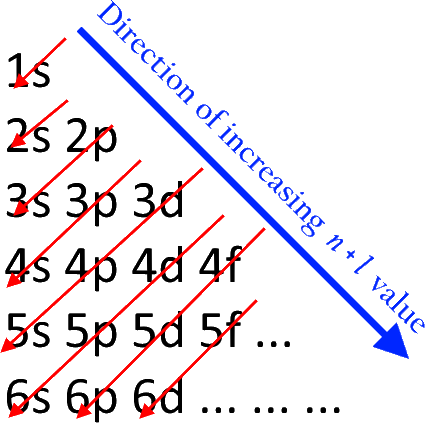
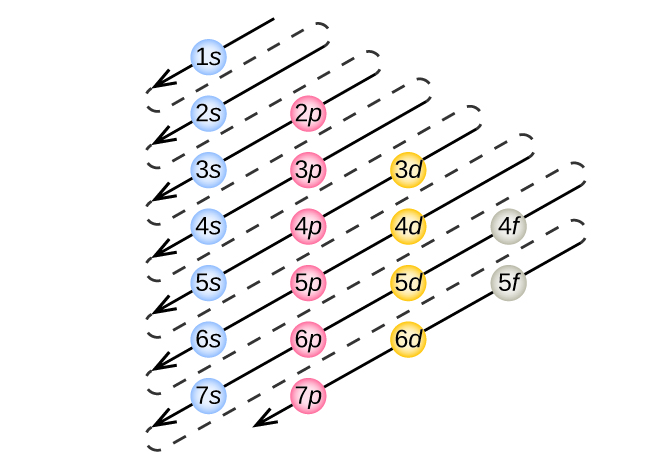
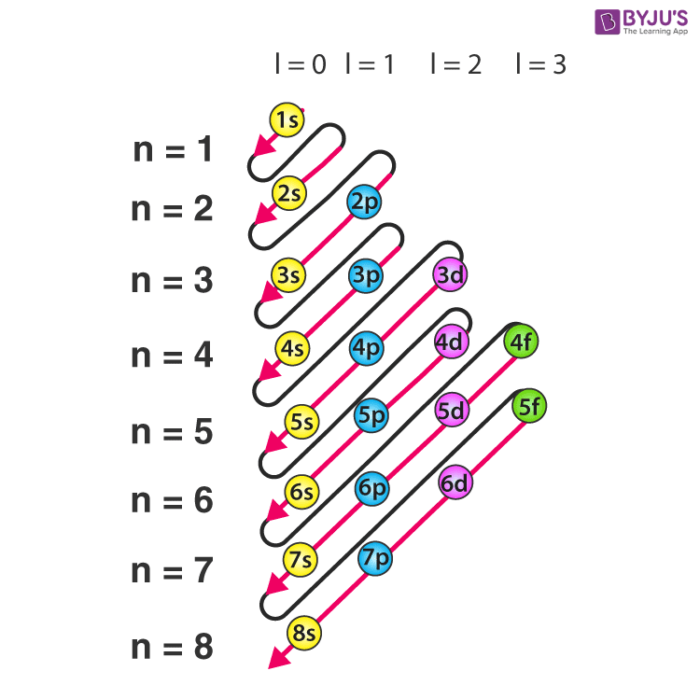
The Aufbau Principle: the (n + l) Rule. We’ve all seen and use the so-called Aufbau Diagram (Figure 1). It is a mnemonic used to remember the order of “filling” of atomic orbitals during the construction of the ground state electron configurations of the elements. The presentation of this diagram is largely disconnected from any physical ...
14.08.2020 · The Aufbau Principle To determine the electron configuration for any particular atom, we can “build” the structures in the order of atomic numbers. Beginning with hydrogen, and continuing across the periods of the periodic table, we add one proton at a time to the nucleus and one electron to the proper subshell until we have described the electron configurations of all …
What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels.
What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels.
What is the basis for exceptions to the Aufbau diagram?Get the worksheet from here: https://madecalculators.blogspot.com/2020/11/chemistry-more-questions.html
What elements are exceptions to the Aufbau principle? For example, ruthenium, rhodium, silver and platinum are all exceptions to the Aufbau principle because of filled or half-filled subshells. In the lower atomic numbers, the difference in energy levels for the normal sequence of electron shells is larger and exceptions are not as common.
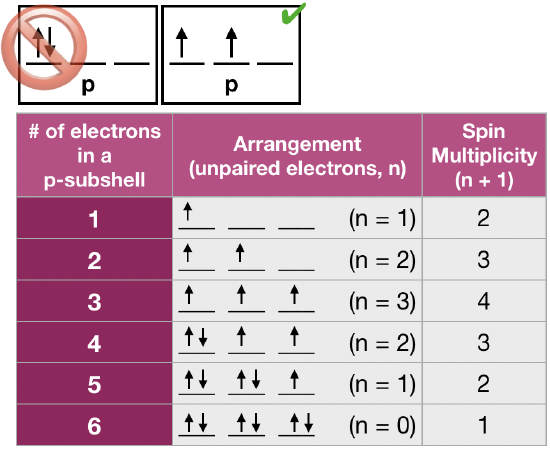
What is the basis for exceptions to the aufbau diagram? Filled and half-filled energy sublevels are more stable than partially-filled energy sublevels. Which scientist developed the quantum mechanical model of the atom? Erwin Schrodinger. The quantum mechanical model of the atom..
What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels.
What is the basis for exceptions to the aufbau diagram In depth view into 20 Year Treasury Rate including historical data from 1993, charts and stats. The exceptions were Belgium and Germany, with shares of more than 20 %. For extra EU trade, the statistical information is mainly provided by the traders on the basis of customs declarations.
aufbau principle. tendency of electrons to enter orbitals of lowest energy first. electron configuration. ... What is the basis for exceptions to the aufbau diagram? Filled and half-filled energy sublevels are more stable than partially-filled energy sublevels.
What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels.
What is the basis for exceptions to Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, ...
What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels. ...
What is the basis for exceptions to the aufbau principle? Filled and half-filled energy sublevels are more stable than partially-filled energy sublevels. He theorized that electrons traveled in...
Exceptions The electron configuration of chromium is [Ar]3d 5 4s 1 and not [Ar]3d 4 4s 2 (as suggested by the Aufbau principle). This exception is attributed to several factors such as the increased stability provided by half-filled subshells and the relatively low energy gap between the 3d and the 4s subshells.
Start studying chem 5. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
What is the basis for exceptions to the aufbau diagram? answer choices . Filled and half-filled energy sublevels are more stable than partially-filled energy sublevels. Electron configurations are only probable. Electron spins are more important than energy levels in determining electron configuration. Some elements have unusual atomic orbitals
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas.The rule is especially applicable to carbon, nitrogen, oxygen, and the halogens, but also to metals such as sodium or magnesium.
The Aufbau principle states that electrons must be added to elements and ions in a VERY specific order with the lowest energy level being filled first and the highest last.
The energy level diagram below shows sublevels to as high as the energy level of the 5f orbitals. Sublevels actually continue to higher energies than this, but 5f is a suitable place to leave an introductory description. Naming the Subshells/Sublevels. Electron sublevels are known by the letters s, p, d, and f. So, for example, electrons in the s sublevel of shell 3 have a different …
The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and superscript number of electrons. The Aufbau Principle. To determine the electron configuration for any particular atom, we can “build” the structures in the order of atomic numbers. Beginning with hydrogen, and continuing across the ...
16.12.2021 · Hence, the molecular orbital diagram of beryllium chloride would be: The sixteen valence electrons are filled in molecular orbitals according to the Aufbau principle, which follows Pauli’s exclusion principle and Hund’s rule. Conclusion. Beryllium chloride is an inorganic compound, which is soluble in various polar solvents. It is a Lewis ...
Which of the following is the correct orbital diagram for a nitrogen (n) atom_ Which of the following is the correct orbital diagram for a nitrogen (n) atom_ Which of the following is the correct orbital diagram for a nitrogen (n) atom_ ...
What is the basis for exceptions to the aufbau diagram? 1 See answer Answer Expert Verified 4.7 /5 4 AnneC Some transition metals don't follow the aufbau, because half-filled orbitals and completely filled orbitals are associated with the stability of the element. For example, For Cu with Z=29
The aufbau principle, from the German Aufbauprinzip (building-up principle), also called the aufbau rule, states that in the ground state of an atom or ion, electrons fill subshells of the lowest available energy, then they fill subshells of higher energy. For example, the 1s subshell is filled before the 2s subshell is occupied. In this way, the electrons of an atom or ion form the most ...
what is the basis for exceptions to the aufbau diagram? A. filled and half filled energy sublevels are more stable than partially filled energy sublevels. B. electron configurations are only probable. C. electron spins are more important than energy levels in determining electron configuration. D some elements have unusual atomic orbitals
Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.. An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons …
What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels.
What is the basis for exceptions to the aufbau diagram? A. filled and half-filled energy sublevels are more stable than partially-filled energy sublevels B. electron configurations are only probable C. electron spins are more important than energy levels in determining electron configuration D. some elements have unusual atomic orbitals
The basis of this prediction is a rule known as the aufbau principle, ... Most of the exceptions to the electron configuration predicted from the aufbau diagram shown earlier therefore occur among elements with atomic numbers larger than 40. Although it is tempting to focus attention on the handful of elements that have electron configurations that differ from those predicted with …
Question: What is the basis for exceptions to the Aufbau principle? The Aufbau Principle The Aufbau Principle gives the rule for the position of electrons in orbitals. It states that electrons fill...
What is the basis for exceptions to the Aufbau diagram? What political value did the Constitution's framers give to the concept of individual liberty? Who is known as the father of modern criminology? What is an example of cultural diversity in sociology? When was the Internet developed? When should sources be cited in your speech quizlet?
Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell.According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels.
What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels.



























0 Response to "45 what is the basis for exceptions to the aufbau diagram"
Post a Comment