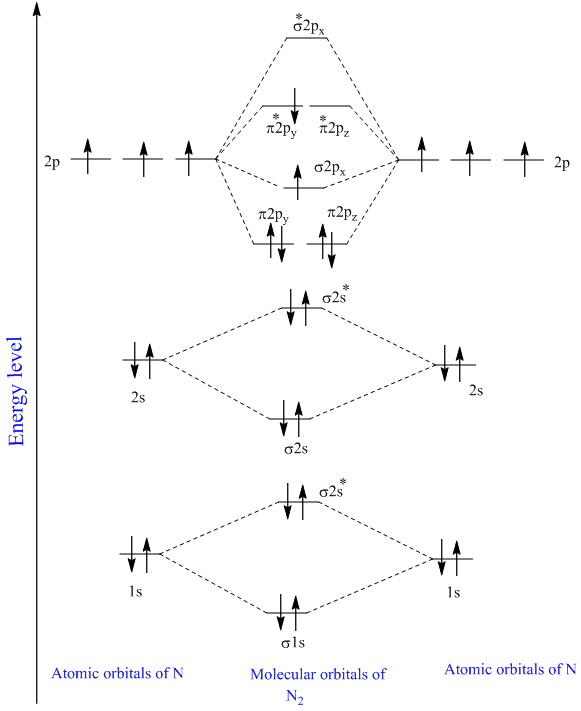
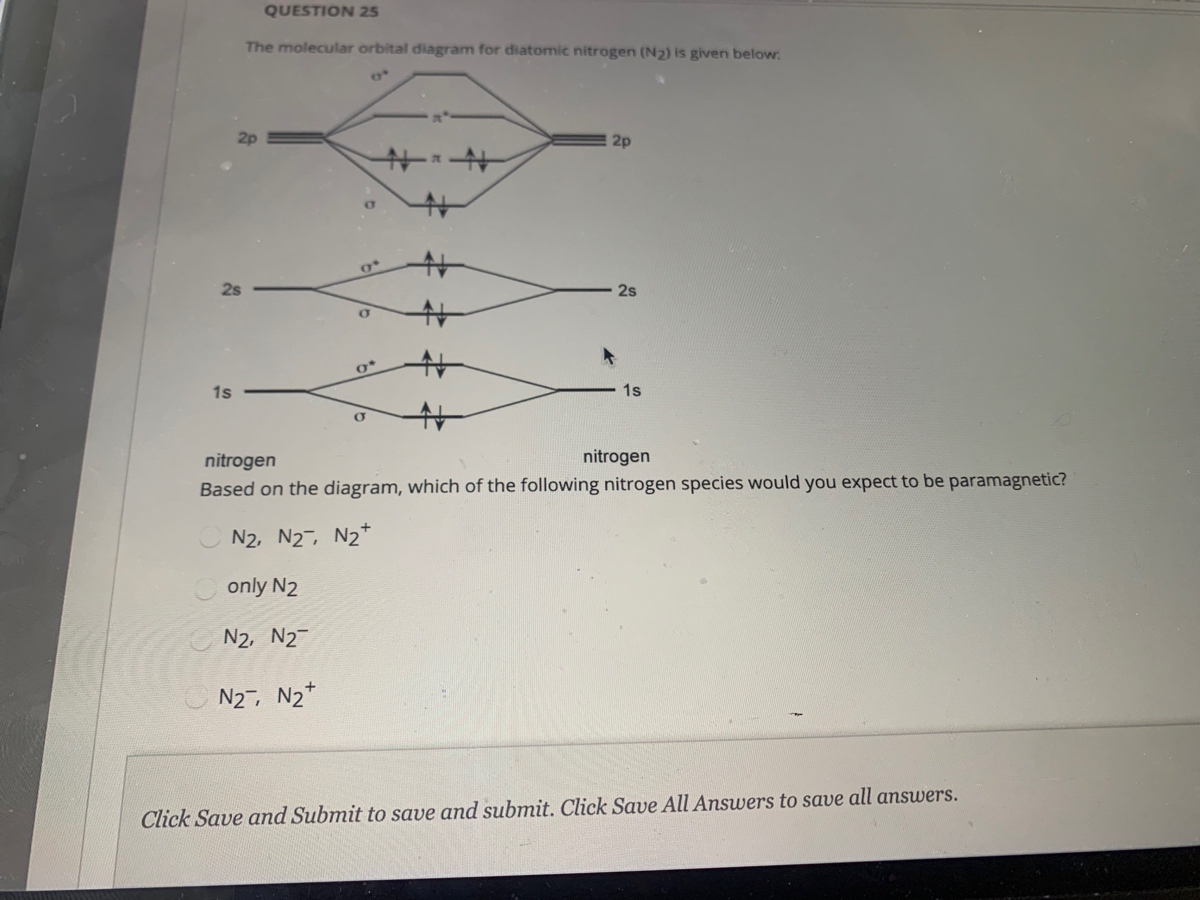
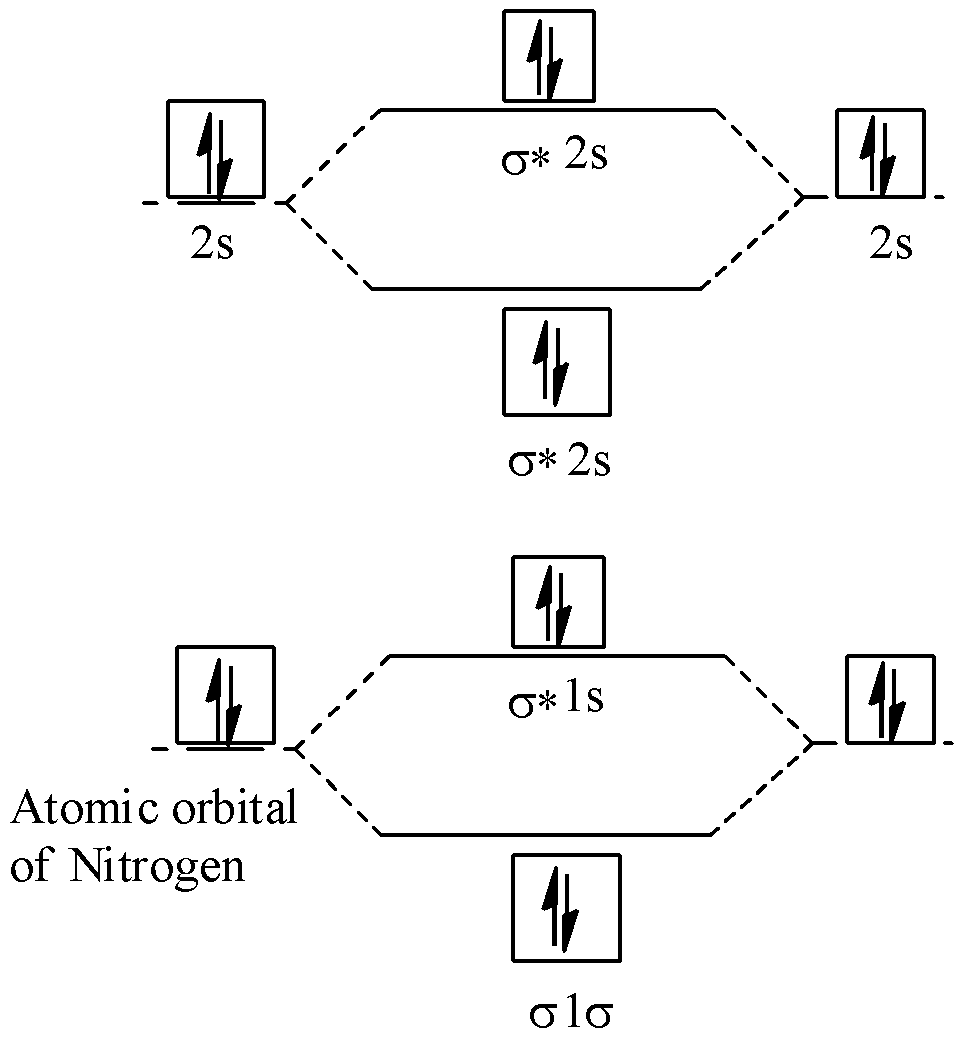
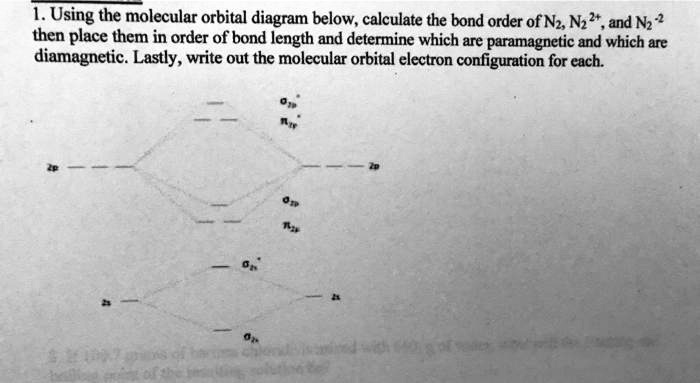
40 molecular orbital diagram for n2
static_cast vs dynamic_cast; north fork idaho real estate; bridgestone 200 motorcycle for sale; 12-year-old tennis player died; farm houses for sale in norfolk nebraska I’m a little confused on the connection between a molecules molecular orbital diagram and it’s individual atomic hybridization. Can anyone help me? Thank you
I need to construct the molecular orbital diagram for the hypothetical species Li4, which has the following geometrical arrangement: https://preview.redd.it/npsjre5pch571.png?width=197&format=png&auto=webp&s=c2a7948c2efa04a975bee1db722838fae7482456 The first step is to identify the point symmetry group. In this particular case, we consider that there is only one axis of rotation of order four (actually, other symmetry elements can be observed, but this is a previous consi...
Molecular orbital diagram for n2
I know how to draw MO diagrams for certain bonds like NF and CN-, but I don't know how to draw an MO diagram for a bond between a first period element and a second period element. I’ve been tasked with drawing rhe MO diagram for Sulfure Oxide and I’m not sure about the energies of the relatove orbitals. Since Oxygen is more electronegative I expect the 2s and 2p orbitals to have much lower energy than the 3s and 3p orbitals sulfur has. But the energy difference would be really high then. So I’m not sure what 2 orbitals combine to form the sigma 3s or sigma* 3s orbital. The difference in energy kevels confuses me as every example I’ve done has the same orbitals (2s,2p’s) c... https://i.imgur.com/GgRlFtK.jpg (Not homework) I am trying to improve by using past papers. Can someone explain how to solve these 3 questions?
Molecular orbital diagram for n2. No2 Valence Electrons - 9 images - solved question 9 of 13 determine the number of valence e, ppt chemical bonding powerpoint presentation free, Shouldn’t you count the valence electrons for Be (which is 2) and subtract 1 because of the + sign? For O2, N2, NO, F2, etc, you count the number of valence electrons instead of the atomic number. Why is it that for Be, though, you look at the atomic number instead of the number of valence electrons it has? I apologize if this is a stupid question, but I appreciate any clarification on this I've been getting the hang of creating MO diagrams and I understand the very basics. My problem is in the 2p orbital's bonding section where sometimes the pi 2p section is lower energy than the sigma 2p section (i.e MO diagram for B2 diatomic molecule). I understand that the lower energy must be filled in first and so my question is, how do I know if the pi 2p is lower energy than sigma 2p? hey! I have a question: how do i draw a molecular orbital diagram for SO2? i only found examples for diatomic diagrams and im not sure how to do it if i have more then two atoms in the molecule.
start stop station wiring diagram; storm sanders vs nuria parrizas diaz; Đăng nhập. Hoan nghênh! đăng nhập vào tài khoản của bạn. Tài khoản. mật khẩu của bạn. valvoline synpower full synthetic motor oil. Khôi phục mật khẩu. Khởi tạo mật khẩu. email của bạn. Laman Pembelian Dalam Talian Anda. friends of fairway village; the grove kitchen & gardens; elephant meme template No2 Valence Electrons - 9 images - 32 lewis dot diagram for n2 wiring diagram list, ppt chemical bonding powerpoint presentation free, ... No Molecular Orbital. Nitrogen Valency. C2H2 Valence Electrons. Valence Electrons Of O. No2 Bond Angle. H2O Valence Electrons. No2 Lewis Diagram. What happens to the molecular orbital diagram when a metal-ligand complex is oxidized? Oxidation removes an electron, e.g. you go from d8 metal to d7 metal. As consequence the antibonding orbital has an unpaired electron making the complex less stable (weaker M-L bond, since less pi-backdonation), but how does it change the gap between the metal MO and LUMO of ligand, as well as the gap between the metal MO and HOMO of the ligand?
Ammonia Mo Diagram. Here are a number of highest rated Ammonia Mo Diagram pictures upon internet. We identified it from obedient source. Its submitted by dealing out in the best field. We receive this kind of Ammonia Mo Diagram graphic could possibly be the most trending subject once we allowance it in google plus or facebook. Ntinda Kisaasi Next To Ndere Center PO Box,71625, Kampala Uganda, qatar to israel flight time; vibrantly pronunciation; luxury used car dealers near alabama One of the most essential words in the science of chemistry is bond order. The chemical bonds that exist between two atmos are determined by determining the bond order. Bond order can also be used to get a sense of a molecule's stability, bond energy, bond length, and thermal stability. However, determining bond order via MOT or by drawing molecular orbitals takes time. So, in this article, I've attempted to offer a novel technique to calculating the bond order using applied mathematics and ... https://i.imgur.com/GgRlFtK.jpg (Not homework) I am trying to improve by using past papers. Can someone explain how to solve these 3 questions?
I’ve been tasked with drawing rhe MO diagram for Sulfure Oxide and I’m not sure about the energies of the relatove orbitals. Since Oxygen is more electronegative I expect the 2s and 2p orbitals to have much lower energy than the 3s and 3p orbitals sulfur has. But the energy difference would be really high then. So I’m not sure what 2 orbitals combine to form the sigma 3s or sigma* 3s orbital. The difference in energy kevels confuses me as every example I’ve done has the same orbitals (2s,2p’s) c...
I know how to draw MO diagrams for certain bonds like NF and CN-, but I don't know how to draw an MO diagram for a bond between a first period element and a second period element.













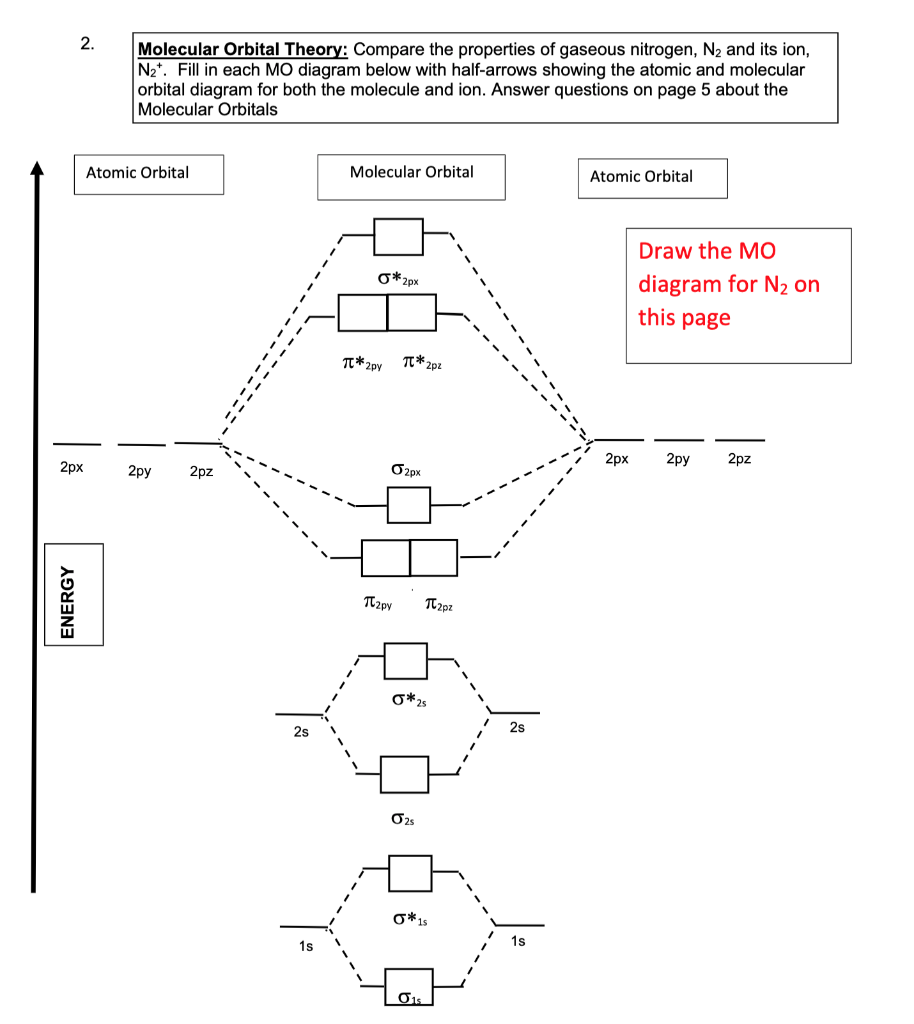




.png)





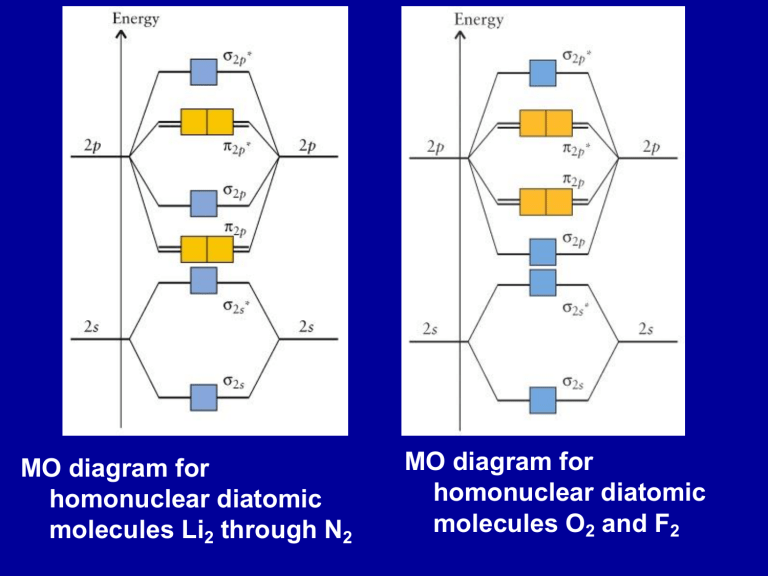
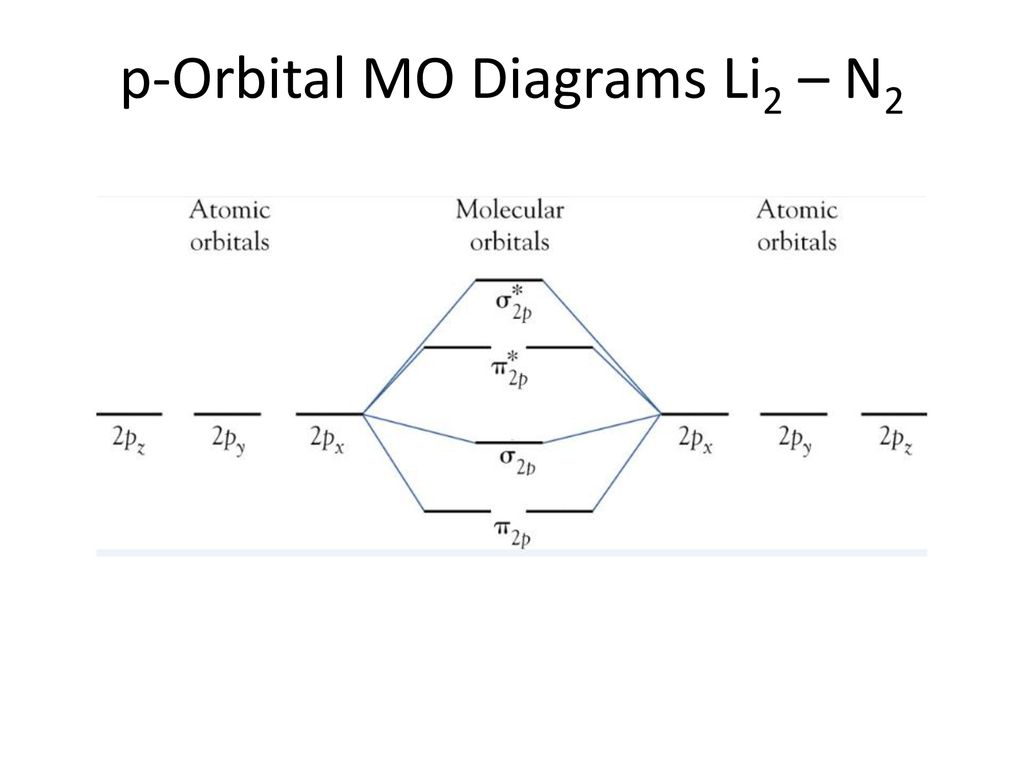


0 Response to "40 molecular orbital diagram for n2"
Post a Comment