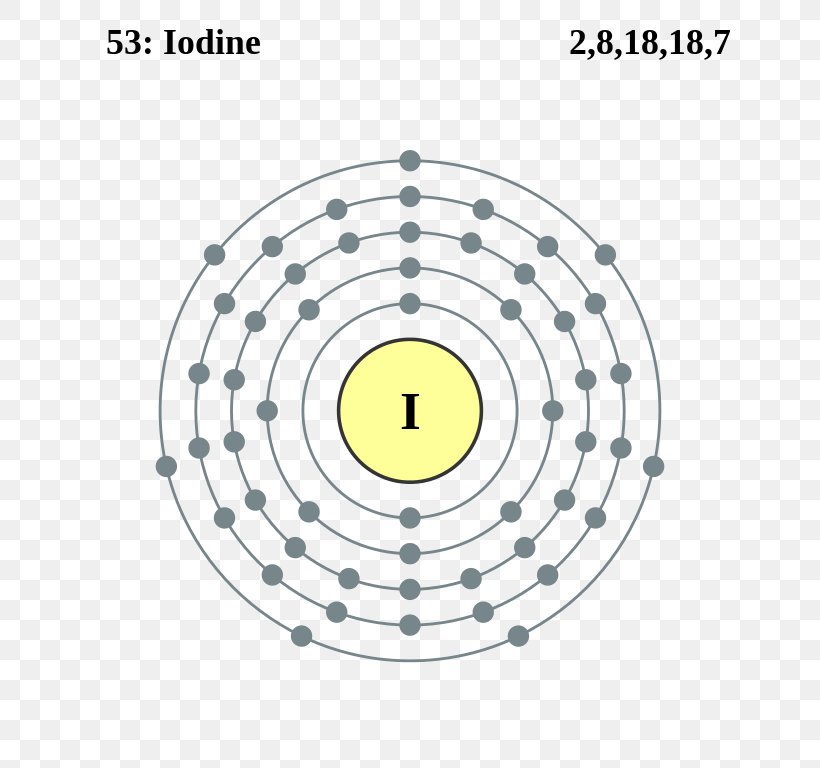
41 electron dot diagram for iodine
Hint: The electron dot diagram is the diagram which clearly shows the bonding between the atoms of a molecule along with the lone pairs of electrons, if they exist in the molecule. Iodine is the molecule consisting of two atoms of iodine bonded together satisfying both the planes. Complete answer: Let us study the concept; A step-by-step explanation of how to draw the I2 Lewis Dot Structure (Iodine Gas).For the I2 structure use the periodic table to find the total number of val...
Iodine Lewis Dot Diagram. Here are a number of highest rated Iodine Lewis Dot Diagram pictures upon internet. We identified it from obedient source. Its submitted by dispensation in the best field. We agree to this kind of Iodine Lewis Dot Diagram graphic could possibly be the most trending subject gone we share it in google lead or facebook.

Electron dot diagram for iodine
Aug 22, 2018 · on Iodine Electron Dot Diagram. Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model . Lewis dot structures help predict molecular geometry. This example Total valence electrons to be "happy" = 1 iodine (8) + 3 chlorine (3 x 8). Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium . Where are the Electrons? Write the full electron configuration, short-hand electron configuration, and fill in the orbital diagrams, for the following elements. 1. Nitrogen ... Complete the electron dot structures below to show how beryllium fluoride (BeF 2) is formed. Use the diagram on page 203 as a model. Be F F Be F F It is the electrostatic force of attraction that binds oppositely charged ions. For students using the Foundation edition, assign problems 1–3, 5–8, 10, 11, 13, 14. anions neutral
Electron dot diagram for iodine. At STP, iodine, I2, is a crystal, and fluorine, F2, is a gas. Iodine is soluble in ethanol, forming a tincture of iodine. A typical tincture of iodine is 2% iodine by mass. Draw a Lewis electron-dot diagram for a molecule of I2. 23)Draw a Lewis electron-dot diagram for a molecule of phosphorus trichloride, PCl3 The electron configuration for O 2 – is 1σ g 2 1σ u 2 2σ g 2 2π u 4 2π g 3. This leaves 1 unpaired electron and gives a bond order of 1.5. (b) Use the same MO diagram as in (a), giving an electron configuration for O 2 + of: 1σ g 2 1σ u 2 2σ g 2 2π u 4 2π g 1. This leaves 1 unpaired electron and gives a bond order of 2.5. Its electron dot diagram resembles that of hydrogen, except the symbol for lithium is used: ... iron, cobalt, nickel, copper, zinc, molybdenum, selenium, and iodine. Minerals are also obtained from the diet. Interestingly, most minerals are consumed in ionic form, rather than as elements or from covalent molecules. Like vitamins, ... Electron Arrangements Name There are three ways to indicate the arrangement of electrons around an atom: 1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1.
Iodine Electron Dot Diagram. which is the correct electron dot diagram for iodine this site might help you re which is the correct electron dot diagram for iodine plz with a pic or if u cnt wit o a pic jus tell me how it electron dot diagram for iodine imageresizertool electron dot diagram for iodine moreover new website periodic table as well as watch along with 9 further also 140 explain ... An electron dot diagram can show you that the symbols for an element surrounded by dots. Each dot stands for one valence electron. How many dots are shown in the electron dot diagram of iodine? HI is a very easy lewis structure to draw due to its simplicity. HI lewis structure. There are only one hydrogen atom and one iodine atom in HI molecule. In the lewis structure of HI, hydrogen atom has made a single bond with iodine atom. Steps of drawing lewis structure of HI. When we draw a lewis structure, there are several steps to follow. A step-by-step explanation of how to draw the I Lewis Dot Structure.For the I structure use the periodic table to find the total number of valence electrons ...
For Iodine we have 7 valence electrons, and 7 for the Chlorine; total of 14 valence electrons for the ICl Lewis structure. We'll put the Iodine here, and the Chlorine right next to it. iodine w/ six electrons around it, bromine w/ six electrons around it and two electrons between them. that will maintain an octet around both atoms. The iodine atom from group vii has 7 valence electrons. The lewis dot diagram for platinum is a diagram showing bonds electrons of the platinum atom within a molecule. That means it has 7 valence electrons so we have 7. Iodine is in group 7 of the periodic table. Lewis Dot Diagram Iodine Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model . The left diagram shows a Lewis dot structure of sodium with. Likewise, they can be used .. The left shows an iodine atom with one lone pair. When we write the . Powered by FlexBook® textbook Platform ® © CK-12 Foundation 2021; Please wait... Please wait...
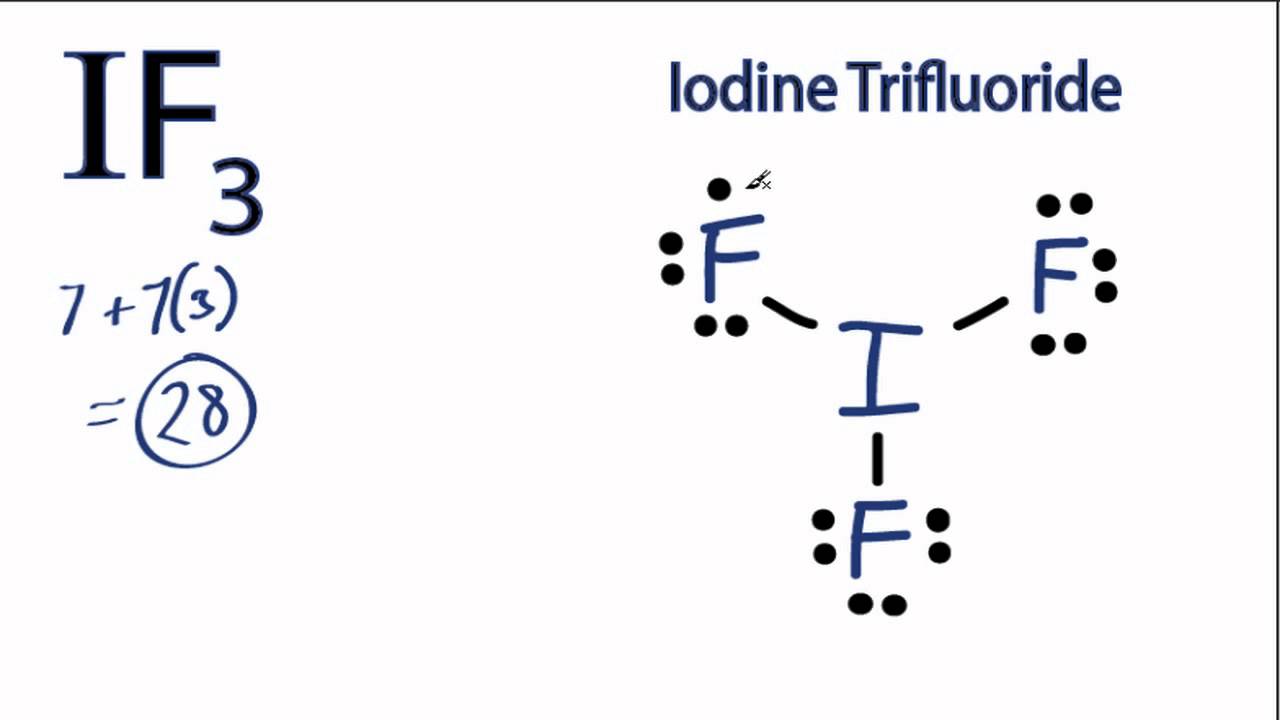
So let's start out. Iodine is in group 7 of the periodic table. That means it has 7 valence electrons, so we have 7. But we have two Iodine atoms, so we need to multiply that by two, giving us a total of 14 valence electrons.
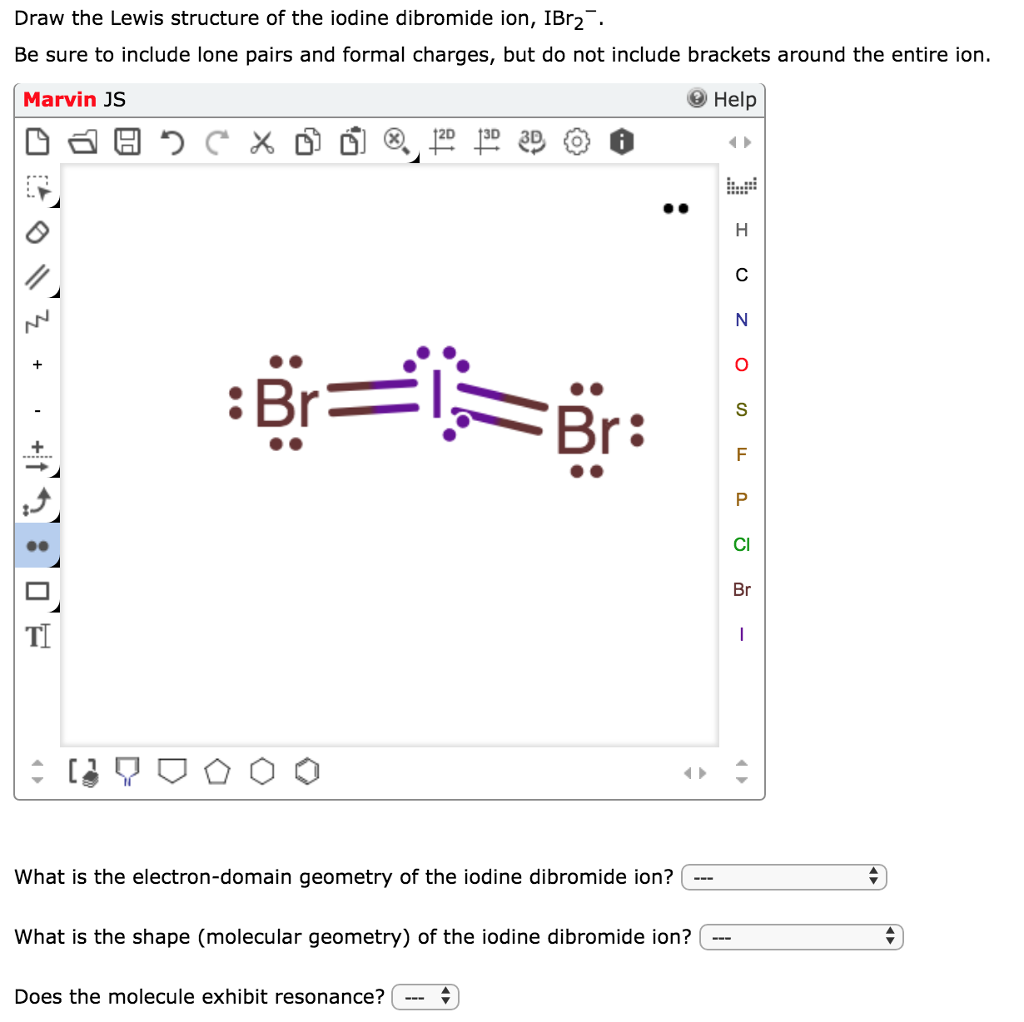
ICl2- lewis structure contains one iodine atom at the middle position whereas two chlorine atoms at the surrounding position. There are three lone pairs present on the central atom of ICl2- lewis structure. Also, the iodine central atom in ICl2- lewis structure violates the octet as it is holding more than 8 electrons in its octet shell.
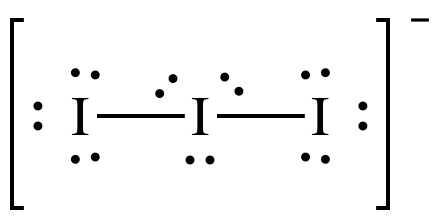
Also, iodine is in the seventh group of the periodic table and has seven valence electrons in its outer orbit. We have three molecules of iodine here which along with an extra electron which gives it a negative charge. So the total number of valence electrons are : 7×3 + 1= 22. There are 22 valence electrons in total in this molecule.
Hydrogen electron configuration is 1s 1.Hydrogen is a s-block element. This article gives an idea about the electron configuration of hydrogen, period and groups, valency and valence electrons of hydrogen, bond formation, compound formation, application of different principles.. The first element of the periodic table is hydrogen and its position at the beginning of the periodic table.
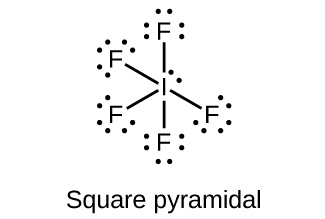
Drawing the Lewis Structure for IF 5. Video: Drawing the Lewis Structure for IF 5. Iodine is below Period Two on the periodic table so it can have an expanded octet (hold more than eight valence electrons). In the Lewis structure for IF5 you'll need to put a total of 12 valence electrons on the Iodine atom in order to draw the Lewis structure.
Since Iodine (I) is below Period 3 on the periodic table it can hold more than 8 electrons. In the Lewis structure for ICl4- the Iodine atom has 12 valence electrons. Also note that you should put the ICl4- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.
Electron Dot Diagram for Iodine. which is the correct electron dot diagram for iodine plz with a pic or if u cnt wit o a pic jus tell me how it looks like electron dot diagram for iodine imageresizertool electron dot diagram for iodine moreover new website periodic table as well as watch along with 9 further also 140 explain using dot and cross in
Electron Dot Diagram for Iodine. which is the correct electron dot diagram for iodine plz with a pic or if u cnt wit o a pic jus tell me how it looks like electron dot diagram for iodine imageresizertool electron dot diagram for iodine moreover new website periodic table as well as watch along with 9 further also 140 explain using dot and cross ...
Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. May 04, · A step-by-step explanation of how to write the Lewis Dot Structure for I2 (Iodine Gas).
Iodine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are there in valence shells of iodine atoms. valence electrons given by iodine atoms = 7 * 2 = 14 Total valence electrons = 14 Total valence electrons pairs
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
13.12.2021 · We also have an additional electron to provide the overall negative charge to the I3 molecule. Total number of valence electrons = 3*7+ 1 =21 + 1 =22. 2. Since all the atoms are iodine, one of these plays the role of the central atom. 3. As you can see in the above diagram, the skeletal structure of I3 with the negative charge has been drawn. 4.
What should the electron dot diagram for iodine look like? Chemistry Covalent Bonds Drawing Lewis Structures. 1 Answer Jahan Psyche Oct 24, 2015 Answer link. Related questions. What are lewis dot structures used for? What is the lewis structure for #SO_2#? How do you draw the lewis structure for ions? ...
For Iodine we have 7 valence electrons, and 7 for the Chlorine; total of 14 valence electrons for the ICl Lewis structure. We'll put the Iodine here, and the Chlorine right next to it. We have a total of 14 valence electrons. We'll put 2 between atoms to form the chemical bond, and we'll go around the outside.
The ground state electronic configuration of Iodine is [Kr] 4d105s25p5. There is only one unpaired electron but we need three unpaired electrons for the formation of three bonds with three chlorine atoms. Hence, one of the electrons from the 5p orbital will promote to 5d orbital for the formation of three bond pairs with three chlorine atoms.
Concentrated solution of potassium iodide and. Ioduro / iodine by Lewis Ford Lewis Structure - Wikipedia, free encyclopedialewis structures (also known as Lewis Dot diagrams, electronic point diagrams. Elements of groups from 15 to 18, such as phosphorus, sulphur, iodine and xenon. Lewis Structures for
Electron Dot Diagram For Iodine Wiring Diagram images that posted in this website was uploaded by Speedtest.illinois.gov. Electron Dot Diagram For Iodine Wiring Diagram equipped with a HD resolution 1280 x 720.You can save Electron Dot Diagram For Iodine Wiring Diagram for free to your devices.. If you want to Save Electron Dot Diagram For Iodine Wiring Diagram with original size you can click ...
Iodine is a diatomic molecule so its molecules are paired as i2 iodine has 7 electrons in its outer shell so 1 electron is shared by each atom. To make the electron dot diagram you put the electron symbol and put a dot on one of the sides for. Chemical properties of iodine.
Orbital diagram for central atom after hybridization 5. Lewis Dot Diagram Worksheet - Worksheet Bunny Jul 14, 2021 · Lewis dot diagram worksheet use the bohr models to determine the number of valance electrons. Molecular Orbital Worksheet 1. Write the molecular orbital configuration of H 2 N 2 O 2 and F 2 molecules.
Complete the electron dot structures below to show how beryllium fluoride (BeF 2) is formed. Use the diagram on page 203 as a model. Be F F Be F F It is the electrostatic force of attraction that binds oppositely charged ions. For students using the Foundation edition, assign problems 1–3, 5–8, 10, 11, 13, 14. anions neutral
Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium . Where are the Electrons? Write the full electron configuration, short-hand electron configuration, and fill in the orbital diagrams, for the following elements. 1. Nitrogen ...
Aug 22, 2018 · on Iodine Electron Dot Diagram. Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model . Lewis dot structures help predict molecular geometry. This example Total valence electrons to be "happy" = 1 iodine (8) + 3 chlorine (3 x 8).





/ICl3_LD-56a12a2b3df78cf77268034c.png)









0 Response to "41 electron dot diagram for iodine"
Post a Comment