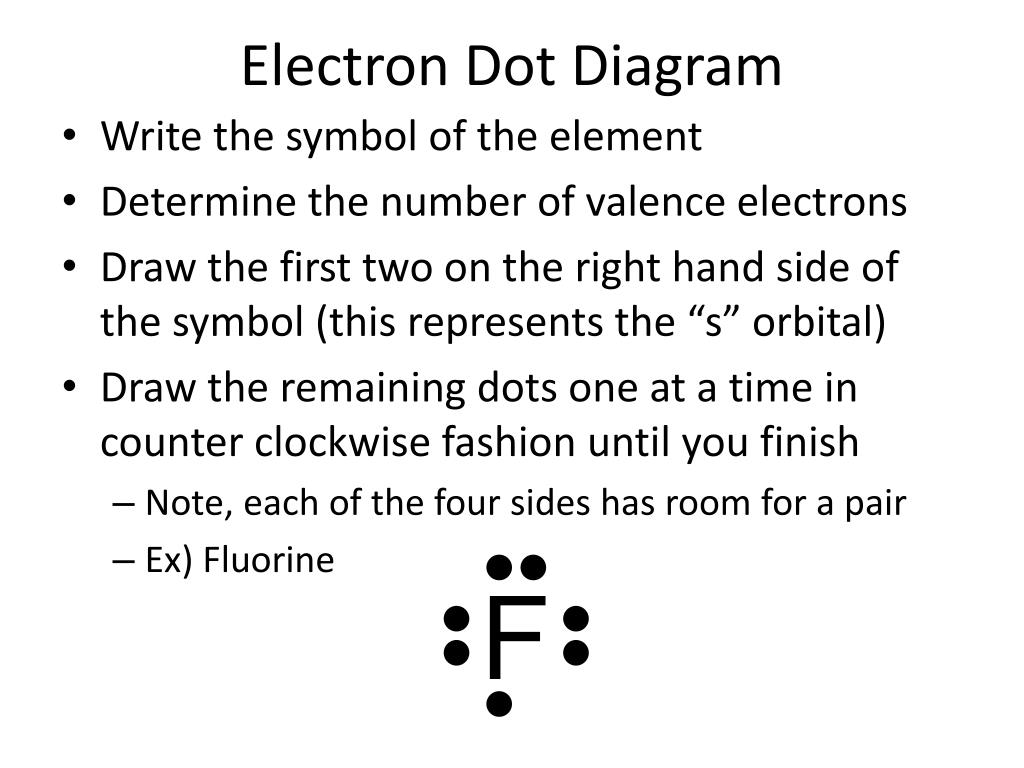
43 electron dot diagram for nitrogen
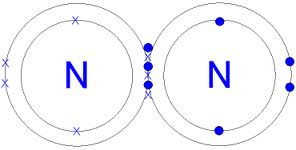
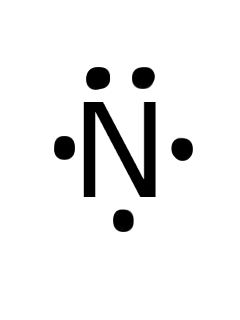
The N2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. What is the electron dot diagram for nitrogen? Note: Nitrogen is in Group 5 (sometimes called Group V or Group 15). Since it is in Group 5 it will have 5 valence electrons. When you draw the Lewis structure for Nitrogen you'll put five "dots" or valance electrons around the element symbol (N).
Electron dot diagram of a Nitrogen atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Nitrogen, we got to know, it has 5 valence electrons. So, just represent these 5 valence electrons around the Nitrogen atom as a dot.

Electron dot diagram for nitrogen
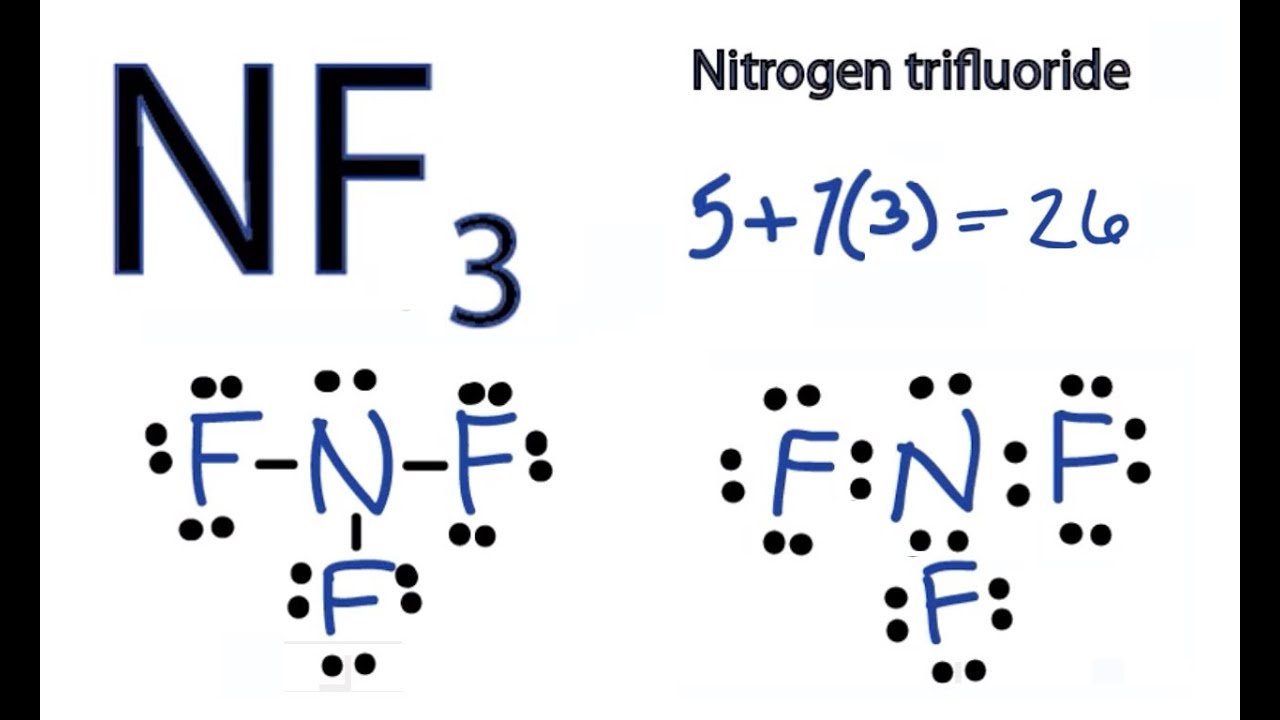
Draw the electron dot structure of Nitrogen molecule [N=7] · Solution · Nitrogen molecule. N=7⇒2K5L N=7⇒2K5L Nitrogen atom shares three electrons forming ...1 answer · Top answer: Nitrogen molecule N = 7 & 2 & 5 & K & L N = 7 & 2 & 5 & K & L Nitrogen atom shares three electrons forming a triple covalent bond. A step-by-step explanation of how to draw the NF3 Lewis Dot Structure (Nitrogen trifluoride).For the NF3 structure use the periodic table to find the total n... A step-by-step explanation of how to draw the Lewis dot structure for N (Nitrogen). I show you where Nitrogen is on the periodic table and how to determine ...
Electron dot diagram for nitrogen. A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t... A step-by-step explanation of how to draw the N3- Lewis Dot Structure.For the N3- Lewis structure use the periodic table to find the total number of valence ... Jan 14, 2022 · Nitrogen dot diagram has a triple bond between two nitrogen atoms. Nitrogen needs to bond itself three times as per octet rule. So two atoms of a same element are connected in pair in Nitrogen dot diagram because nitrogen is diatomic molecule in nature. N2 Dot Structure The three bonds appear as three parallel lines between the N atoms. This is a triple bond. Each connection has two electrons ... The Lewis structure for NH3 is.The Lewis dot structure for NH3 starts with an N atom connected on three sides with a dash, each to an H atom. The N atom then has two dots on the unconnected side. NH3, commonly known as ammonia, is arranged as a T-shaped molecule with nitrogen at its center and three hydrogen atoms at its extremities.
Apr 11, 2016 — The Lewis structure of Nitrogen atom can be drawn if one knows the number of valence electrons of Nitrogen. The electronic configuration of ...1 answer · Explanation: The Lewis structure of Nitrogen atom can be drawn if one knows the number of valence electrons of Nitrogen. The electronic configuration ... Nitrogen (N 2) Molecule Lewis Structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure.. N 2 lewis structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. A step-by-step explanation of how to draw the NI3 Lewis Dot Structure (Nitrogen Triiodide).For the NI3 structure use the periodic table to find the total num... Jun 08, 2020 · Note: Nitrogen is in Group 5 (sometimes called Group V or Group 15). Since it is in Group 5 it will have 5 valence electrons. When you draw the Lewis structure for Nitrogen you'll put five 'dots' or valance electrons around the element symbol (N).
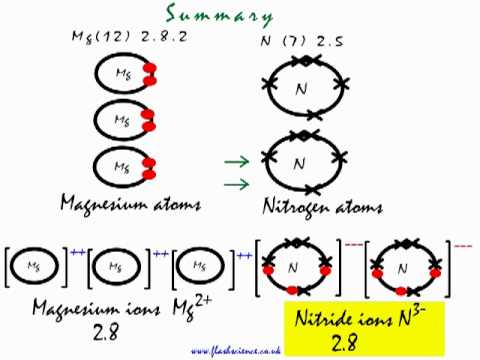
1. Draw an “electron dot” diagram showing the first 18 elements in the periodic table. 2. Explain how the electron dot diagram is similar for families in the periodic table. 3. Draw an electron dot diagram showing the formation of ions and ionic compounds. 4. Explain how hydrogen can be considered as behaving like a metal or a nonmetal. A step-by-step explanation of how to draw the Lewis dot structure for N (Nitrogen). I show you where Nitrogen is on the periodic table and how to determine ... A step-by-step explanation of how to draw the NF3 Lewis Dot Structure (Nitrogen trifluoride).For the NF3 structure use the periodic table to find the total n... Draw the electron dot structure of Nitrogen molecule [N=7] · Solution · Nitrogen molecule. N=7⇒2K5L N=7⇒2K5L Nitrogen atom shares three electrons forming ...1 answer · Top answer: Nitrogen molecule N = 7 & 2 & 5 & K & L N = 7 & 2 & 5 & K & L Nitrogen atom shares three electrons forming a triple covalent bond.
This digitally-colorized, negative-stained transmission electron microscopic (TEM) image depicted some of the ultrastructural morphology of the A/CA/4/09 Swine Flu virus.
This illustration depicts a three-dimensional (3D), computer-generated image of a cluster of barrel-shaped, Clostridium perfringens bacteria. The artistic recreation was based upon scanning electron microscopic (SEM) imagery. See PHIL 21914, for another view of these microbes.
Produced by the National Institute of Allergy and Infectious Diseases (NIAID), this highly magnified, digitally colorized scanning electron microscopic (SEM) image, revealed ultrastructural details at the site of interaction of numerous yellow colored, Middle East respiratory syndrome coronavirus (MERS-CoV) viral particles, located on the surface of a Vero E6 cell, which had been colorized blue.










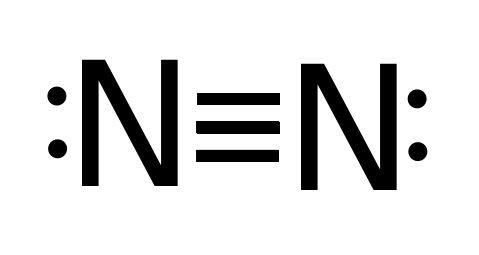


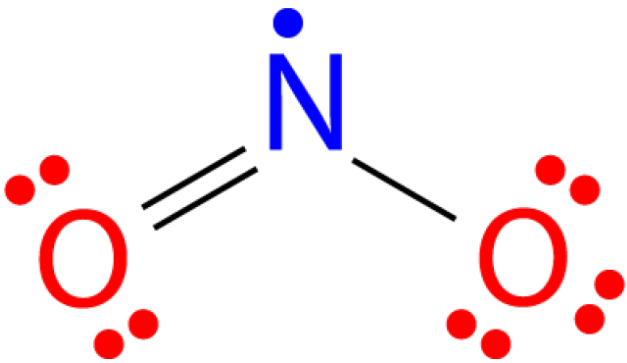
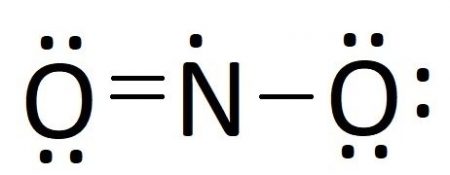

/NO2_Dot-56a12a2c3df78cf772680359.png)








0 Response to "43 electron dot diagram for nitrogen"
Post a Comment