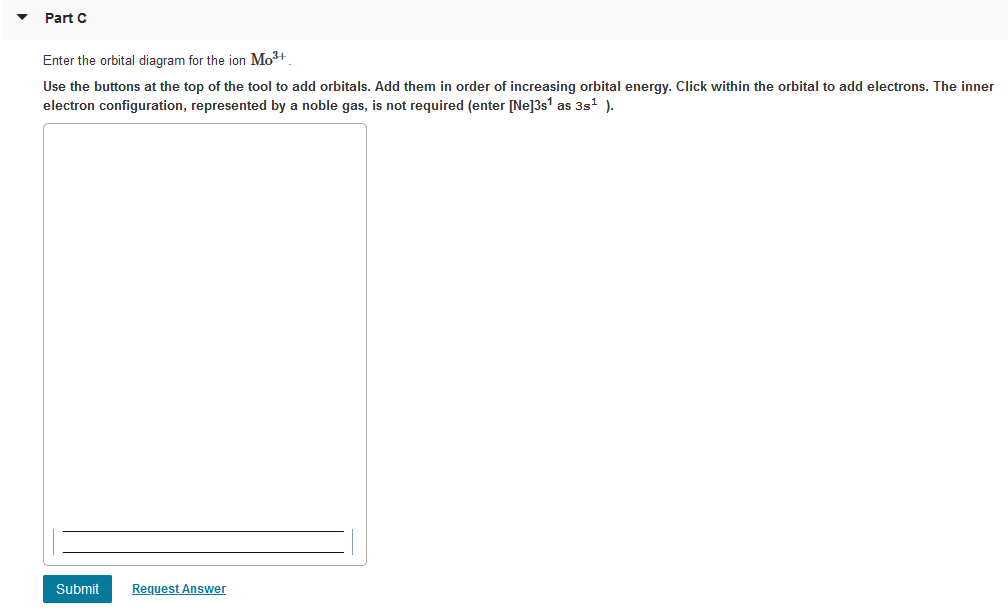
44 enter the orbital diagram for the ion mo3+.
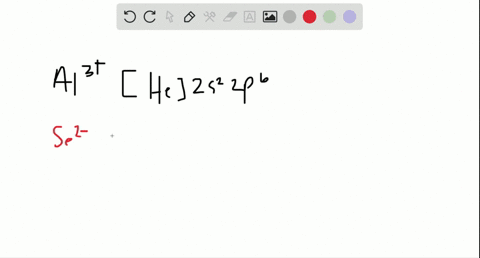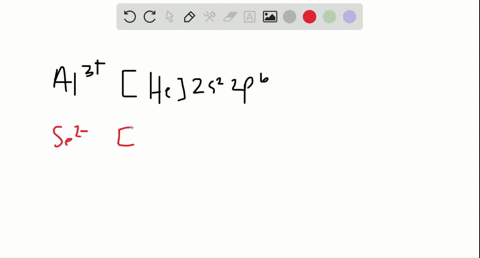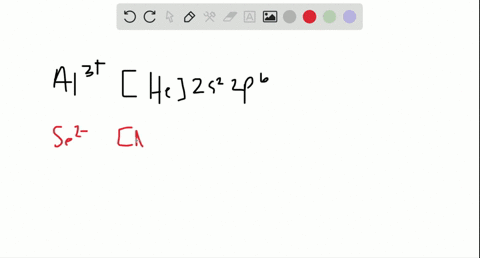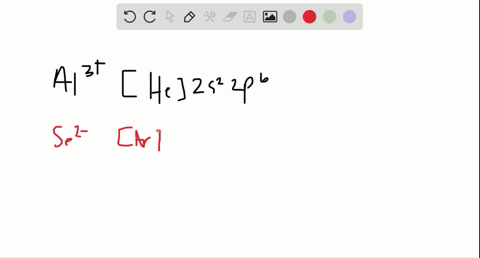
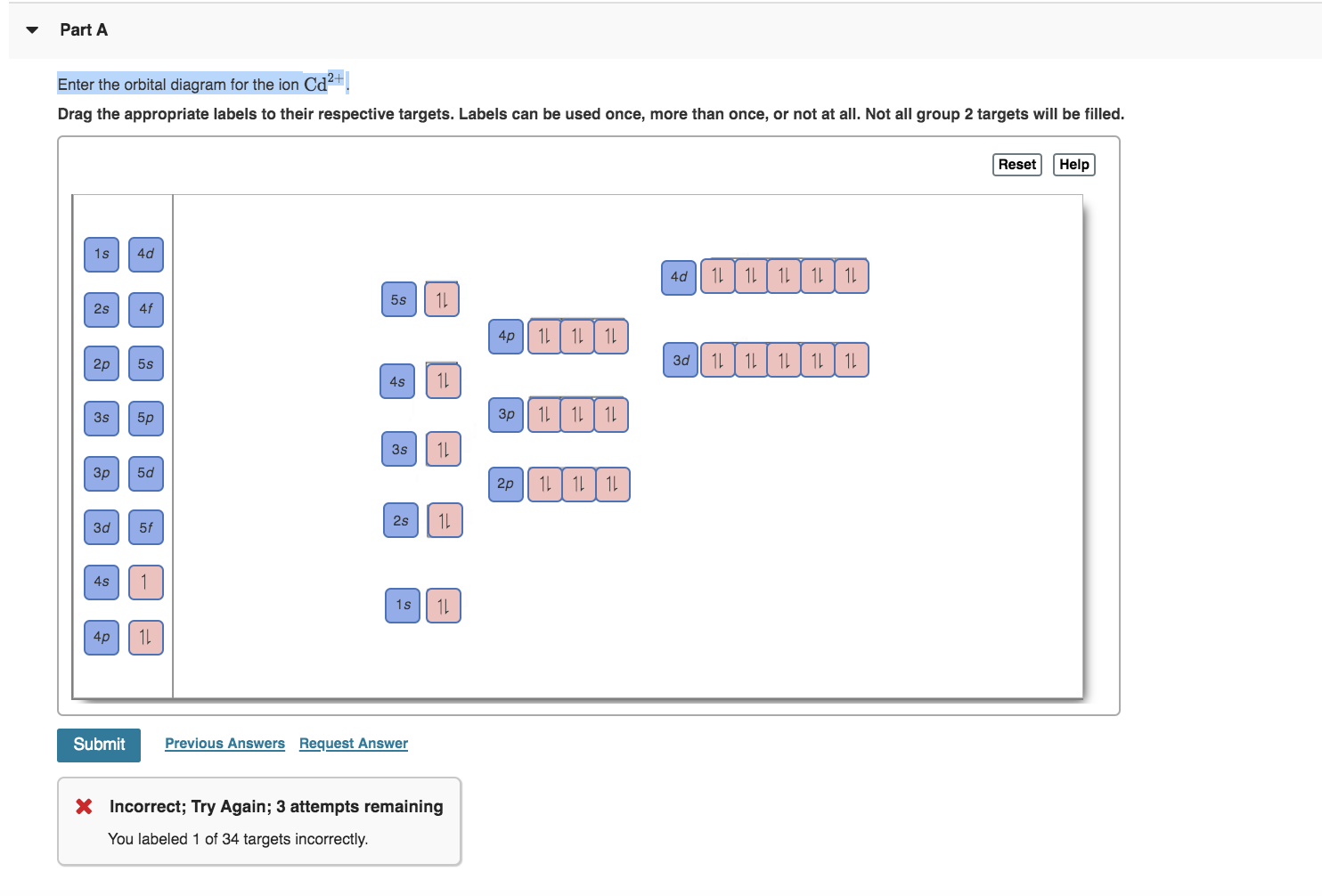
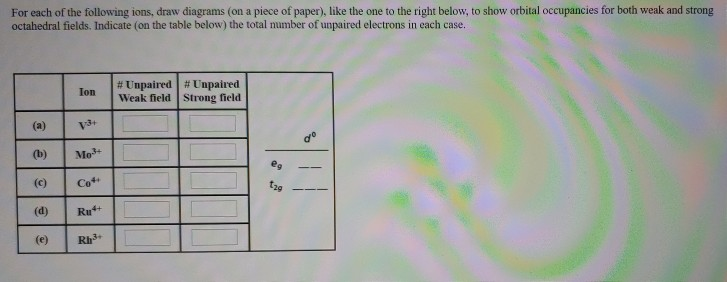
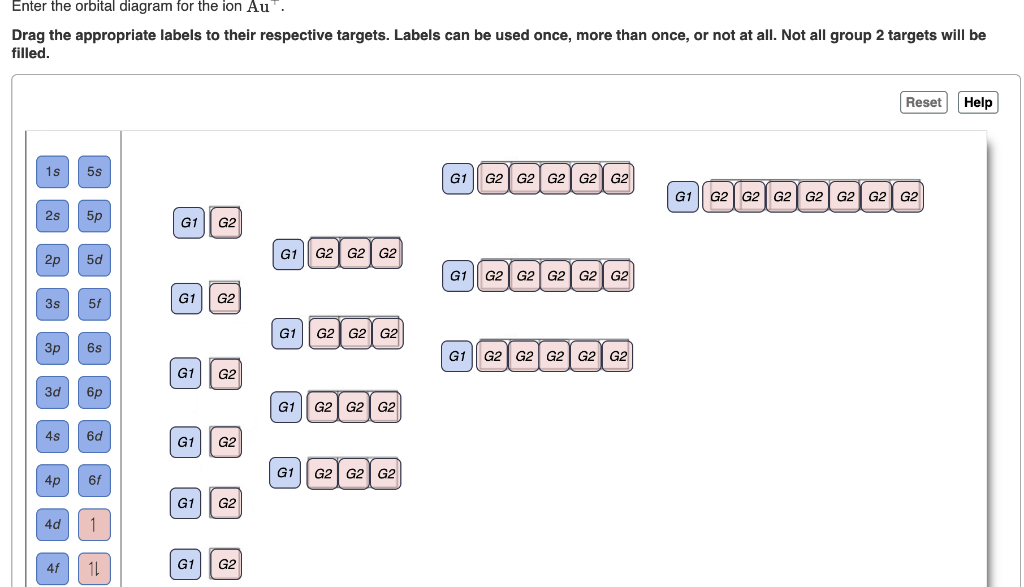
2. Enter the orbital diagram for the ion Au+ 3.Construct the orbital diagram for the ion Mo3+ 4.Construct the orbital diagram for the ion Zr2+ ) Question: 1. Enter the orbital diagram for the ion Cd2+ Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all group 2 targets will be ... Enter The Orbital Diagram For The Ion Mo3+. However, even though the 5s orbital is lower in energy than the 4d orbital, the electrons in the 4d orbitals shield the electron in the 5s orbitals. Mo is element 42 so for the 3+ ion you draw the configuration for . It is not the same orbital diagram as Y this is because it is a transition metal.
This means that it is easier for the electron in the 5s orbital to leave. So, the 5s electron get ionized first. After the 5s electron leave, the next two electrons to be ionized comes from the 4d orbital. Therefore, the electronic configuration of Mo3+ is. [Kr]4d3.
Enter the orbital diagram for the ion mo3+.
To write the configuration for the Molybdenum and the Molybdenum ion, first we need to write the electron configuration for just Molybdenum (Mo). We first n... Enter the orbital diagram for the ion Mo3+ ‣ When an element is a cation (+) you REMOVE electrons. ‣ Electrons are generally removed from the "s" sub-level 1.) Remove 2 electrons from 5s2 2.) Remove 1 electron from 4d4 ANSWER: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d3. Doesn't the 5s come before the 4d? I chose [Kr]5s^2 on a quiz but it was wrong. Therefore, the ground state electron configuration for Zr 2+ is : [Kr]4d 2 5s 2. In^+1 [Kr] 5s2 4d10. 1s2, 2s2, 2p6. 1) nuclear charge and relative energy of 3d and 4s orbitals, 2) relative e-e repulsions in 3d and 4s orbitals, 3) exchange energy. Booster Classes. Need an editable periodic table to edit? Enter the ...
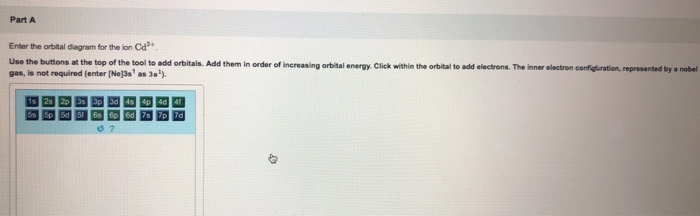
Enter the orbital diagram for the ion mo3+.. Mo: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d4 Mo 3+: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s0 4d3 Mo is 42 on the Periodic Table, since the question asks for Mo3+, you have to subtract 3 electrons. Enter The Orbital Diagram For The Ion Mo3+. Apr 23, 2019 · a) Mo3+: [Kr]4d 3. The electron gained would go into the 2pz orbital and is the second electron in that Y 4d a) The orbital diagram shows the element is in period 4 (n = 4 as .. Watch the video solution for the question: Enter the orbital diagram for the ion Zr2 + .... Subjects . Science ... Q. Write orbital diagram to represent the electron configurations-without hybridization-for F in SF2. Q. Choose the correct orbital diagram for vanadium. See all problems in The Electron Configuration: Ions ... Write orbital diagram for Mo3+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within the orbital to add electrons.
Mo3+ Orbital Diagram. Compact version of orbital energy diagram with each orbital represented . Mo2+, Mo3+, Mo4+ and Mo5+ are all known in aqueous solution. That's why Mo3+ has 39 electrons instead of 42 in its ground state electron The normal electron configuration of zinc is [Ar] 3d 10 4s 2, with 2 4s orbital. Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic. a. C d 2 + Cd^{2+} C d 2 +, b. A u + Au^+ A u +, c. M o 3 + Mo^{3+} M o 3 +, d. Z r 2 + Zr ^{2+} Z r 2 + Explanation. Verified. Step 1 1 of 6. In writing orbital diagrams, first, determine the electron configuration of the neutral atom and remove ... Write the electron configuration for the following ion. V3+ I got 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. In simplified form, I got [Ar]4s^2. I checked my answer against the answer given in the back of my chemistry textbook, and the two didn't correspond. The answer my book gives is [Ar] 3d^2. I don't understand what I did wrong... Answer to Write orbital diagram for Mo3+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing. Write orbital diagram for Mo3+? ground-stste electron configuration of the transition metal ion Mo3+ . Mulitple choice: why is it uncommon to draw lewis diagrams for ionic compounds?
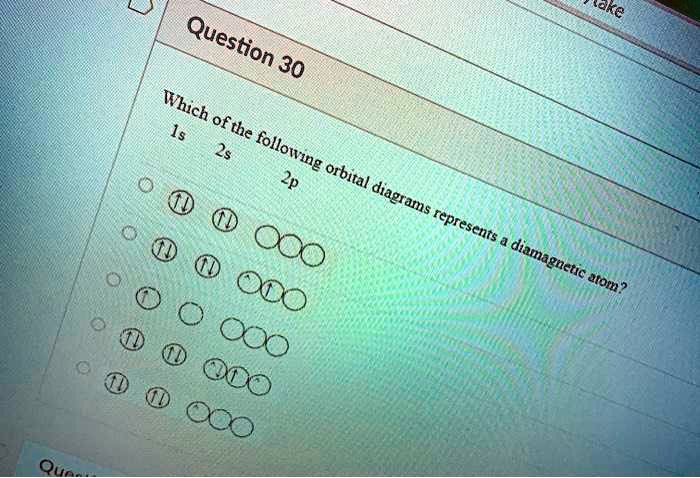
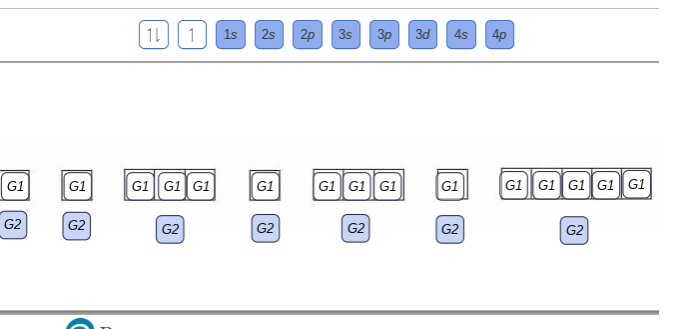
Write orbital diagram for Mo3+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within. Write the condensed ground-stste electron configuration of the transition metal ion Mo3+ .. (16 points) Write the electron configuration for H. (1 point) Write the electron configuration for O. Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. V5+ b. Cr3+ c. Ni2+ d. Fe3+ Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ... Enter the orbital diagram for the ion Cd2+Cd2+. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all group 2 targets will be filled. Reset Help 1s Group 1 1s 4d Group 1 4d 2s Group 1 2s 4f Group 1 4f 2p Group 1 2p 5s Group 1. Question: Enter the orbital diagram for the ion Cd2 ... 88chevy 1500 Ignition Wiring Diagram; Vertical Redraw Of Wiring Diagram; Parrot Bluetooth Ck3000 Wiring Diagram; Cat C12 Ecm Wiring Diagram; Crane Ignition Hi-6rc Wiring Diagram; Holley Dominator Efi Wiring; Enter The Orbital Diagram For The Ion Mo3+. Diagramas De Transmisiones Automaticas; 2001 Ford E450 7.3l Diesel Engine Wiring Diagram ...
Doesn't the 5s come before the 4d? I chose [Kr]5s^2 on a quiz but it was wrong. Therefore, the ground state electron configuration for Zr 2+ is : [Kr]4d 2 5s 2. In^+1 [Kr] 5s2 4d10. 1s2, 2s2, 2p6. 1) nuclear charge and relative energy of 3d and 4s orbitals, 2) relative e-e repulsions in 3d and 4s orbitals, 3) exchange energy. Booster Classes. Need an editable periodic table to edit? Enter the ...
Enter the orbital diagram for the ion Mo3+ ‣ When an element is a cation (+) you REMOVE electrons. ‣ Electrons are generally removed from the "s" sub-level 1.) Remove 2 electrons from 5s2 2.) Remove 1 electron from 4d4 ANSWER: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d3.
To write the configuration for the Molybdenum and the Molybdenum ion, first we need to write the electron configuration for just Molybdenum (Mo). We first n...
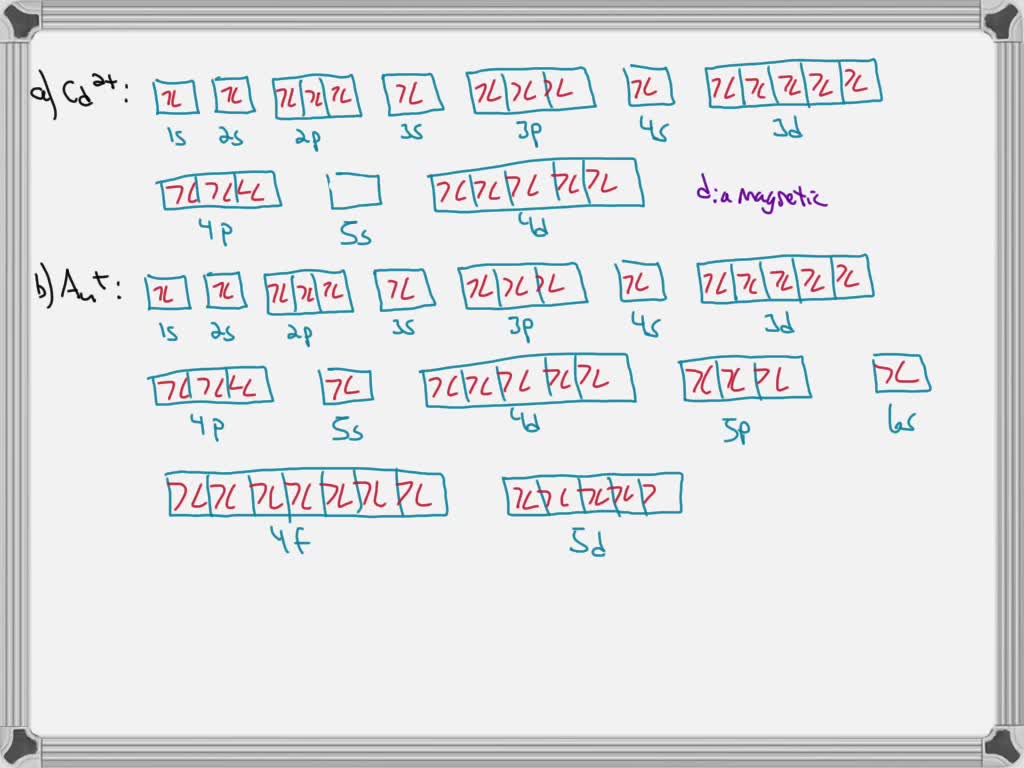
write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic a cd2 b au c mo3 d zr2 2


















![Give the ground state electron configuration for Br -.(A) [Ar]4s23d104p5(B) [Ar] 4s24p6(C) [Ar]4s24p6(D) [Ar]4s24d104p4(E) [Ar]4s23d104p6](https://cdn.clutchprep.com/video_thumbnails/34471.jpg)




0 Response to "44 enter the orbital diagram for the ion mo3+."
Post a Comment