44 oh- molecular orbital diagram
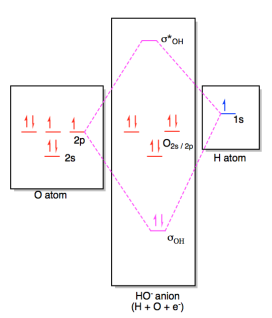
Property Name Property Value Reference; Molecular Weight: 17.007: Computed by PubChem 2.1 (PubChem release 2021.05.07) XLogP3-AA-.6: Computed by XLogP3 3.0 (PubChem release 2021.05.07) The molecular orbital diagram for hydroxide ion is not much more complicated. This molecule is diatomic; it comes from the combination of an oxygen atom with a hydrogem atom, with the addition of an extra electron to provide the negative charge of the ion. In the diagram below, the hydrogen ...
April 11, 2014 - The UIC Department of Chemistry is a Tier-1 Research Institution in Analytical, Biological, Educational, Inorganic, Organic, and Physical Chemistry. Located in downtown Chicago.
Oh- molecular orbital diagram
Consider the hydroxide ion, OH and do the following: a. Prepare a molecular orbital diagram and fill with electrons given the following atomic orbital potential energies: O(2s) = -32.3 eV, O(2p) =-15.8 eV, H(1s) = -13.6 eV. Be sure to label each molecular orbital (bonding, anti-bonding, non-bonding). b. Sketch each molecular orbital's shape in ... Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in 1.The atomic orbital of the metal center and of surrounding ligands combine to form new orbitals, known as molecular orbitals. 2.The number of molecular orbitals formed is the same as that of the number of atomic orbitals combined. 3.The additive overlap results in the bonding molecular orbital while the subtractive overlap results in the
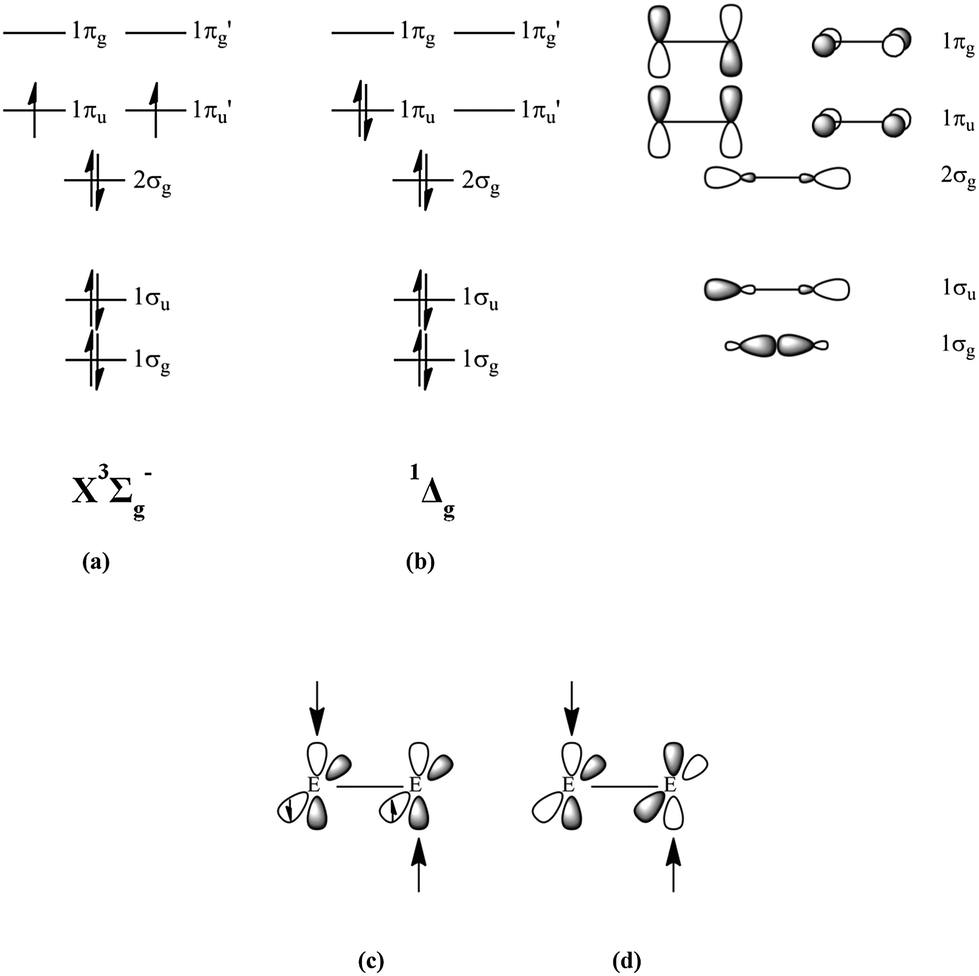
Oh- molecular orbital diagram. Where is the energy difference (Between which orbitals) that corresponds to o (10Dq)? Label o on the MO diagram. What type of orbitals (bonding, non-bonding, antibonding) are the "crystal field" orbitals? Explain why. Calculate the o for [Co(NH3)6]3+, then determine if it is low- or high-spin. Fill in the MO diagram accordingly. The hydroxyl radical is the diatomic molecule • OH.The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals is produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. Core orbitals are omitted. Marks 8 Using arrows to indicate electrons with their appropriate spin, indicate on the above diagram the ground state occupancy of the atomic orbitals of O and H, and of the molecular orbitals of OH. In the provided boxes on the above diagram, label the molecular orbitals as n, σ, σ∗, π, π∗, etc. Molecular orbitals for Octahedral complexes The combination of the ligand and metal orbitals (4s, 4p x, 4p y, 4p z, 3d z2, and 3d x2-y2) form six bonding and six antibonding with a 1g, e g, t 1u symmetries. The metal T 2g orbitals do not have appropriate symmetry - nonbonding Electron in bonding orbitals provide the potential energy that holds ...
May 9, 2021 - Figure 4.11.1: Molecular Orbital Energy-Level Diagram for \(\pi\) Bonding in Ethylene. As in the diatomic molecules discussed previously, the singly occupied 2pz orbitals in ethylene can overlap to form a bonding/antibonding pair of \(\pi\) molecular orbitals. August 15, 2020 - A simple approach to molecular orbital (MO) theory for heterogeneous diatomic molecules is to show the energy level diagram. The MO energy levels can be worked out following these steps: Answer (1 of 2): Here is a useful MO diagram of HCL found on the internet: The Cl electrons residing up to 3s orbital (1s, 2s, 2px,2py,2pz,3s) are largely stabilized than H electron in 1s orbital and therefore they cannot mix and form bond. The 3p electrons of Cl have comparable energy with the ... August 12, 2020 - What we see here is a molecular orbital interaction diagram. The middle of the diagram is just the molecular orbital energy diagram. It is analogous to the atomic orbital energy diagram (which goes 1s, 2s, 2p, 3s...). The order of energy so far is σ1s, σ1s*. The sides of the diagram just ...
Download scientific diagram | Schematic molecular orbital diagram of O 3 and O ϩ 3 illustrating the from publication: High-resolution pulsed-field-ionization zero-kinetic-energy photoelectron ... A molecular orbital (MO) energy level diagram for the OH" radical is shown below. z is the intemuclear axis Outline; in full the reasoning use to derive ...4 answers · Top answer: If the molecule H. O. Follows a very similar molecular orbital diagram as that of H. F. ... Nov 04, 2008 · Molecular orbital of the hydroxyl radical with unpaired electron Skeletal formulae of 1-hydroxy-2(1H)-pyridinethione and its tautomer The hydroxyl radical, OH, is the neutral form of the hydroxide ion. Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most March 1, 2021 - The positions and energies of electrons in molecules can be described in terms of molecular orbitals (MOs) A particular spatial distribution of electrons in a molecule that is associated with a …
Allyl Radical · Compass · Tables · Index · Introduction · Professor Patricia Shapley, University of Illinois, 2012
Dec 30, 2019 · I would like to understand how to create a molecular orbital diagram for the hydroxide ion from scratch. This includes understanding the shape of the molecular orbital. Here is an attempt I have come up with: Where the top MO is sigma* and the bottom is simply sigma. But this makes no sense.
July 17, 2020 - The key difference is that in molecular orbitals, the electrons are allowed to interact with more than one atomic nucleus at a time. Just as with atomic orbitals, we create an energy-level diagram by listing the molecular orbitals in order of increasing energy.
MO diagrams of Heteronuclear Diatomic Molecules |PART-1 | OH-,HF, CN- |By ved Sir | Chem academyhttps://www.youtube.com/channel/UCNPYcBEfxHqTwkxLEw1sHXwThis ...
1- Draw the molecular orbital diagram of transition metal ion in high-spin Mn(H2O)4(OH)2 complex, also determine the number of unpaired electron.1 answer · 0 votes: & Molecules Orbital diagram for Oh ion TALLI 17 Lowest Unoccupied Molecular vebital CLUMO) H-atom Highest Occupied molecular eshital - d ル ル T(m), ...
Oh- molecular orbital diagram FIG. 1: Two molecular orbital s of water that have bonding ˙ OH character, 2a 1 and 1b 1. Their combined contribution leads to 2 single OH bonds: (2a 1)2(1b 1)2!(˙ OH1)2(˙ OH2)2 ˙ orbital s are oriented along the bonds, whereas ˇ- orbital s are oriented perpendicular to the molecular plane.
FIG. 1: Two molecular orbitals of water that have bonding ˙ OH character, 2a 1 and 1b 1. Their combined contribution leads to 2 single OH bonds: (2a 1)2(1b 1)2!(˙ OH1)2(˙ OH2)2 ˙orbitals are oriented along the bonds, whereas ˇ-orbitals are oriented perpendicular to the molecular plane. In a symmetric molecule, orbitals belong to di erent ...
Homepage of the John McGrady's Computational Inorganic Chemistry Group, Inorganic Chemistry Laboratory, South Parks Road, University of Oxford. The group's research interests focus on the electronic structure of inorganic systems in the broadest sense. We apply modern computational methods ...
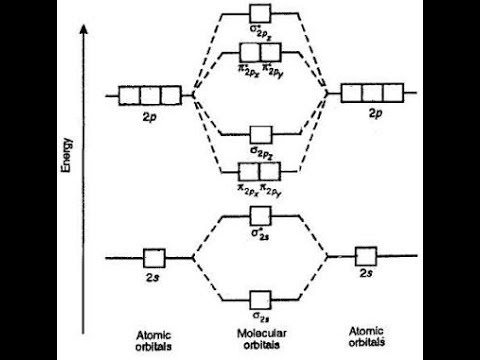
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Molecular Orbitals for Water (H 2 O) The five occupied and the lowest three unoccupied molecular orbitals of the isolated molecule (1a 1) 2(2a 1) 2(1b 2) 2(3a 1) 2(1b 1) 2 were calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set (experimental data is given in [1289]). They are set out with the lowest
Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
Download scientific diagram | Energy level diagram for the molecular orbitals of OH ). H and O atom orbitals, which combine to form the molecular orbitals, are on the left and right side of the figure, respectively. from publication: Kinetics of the Reactions of Water, Hydroxide Ion and Sulfide ...
Show below is a molecular orbital diagram for an octahedral ML6 complex and the Oh character table. From the ligand pi orbitals, only the t2g set is considered. All ligand orbitals are fully filled. Guiding lines connecting atomic orbitals to their corresponding molecular orbitals have been omitted.
Atomic orbital. Mulliken labels. C2v. D3h. D4h. Td. Oh ... MO diagram of homonuclear diatomic molecules ... Molecular Orbital Theory – Walsh diagram.29 pages
September 8, 2021 - Walsh correlation diagram is a plot of molecular orbital energy as a function of some systematic change in molecular geometry. For example, the correlation between orbital energies and bond angle for …
Sigma pi bond formation Orbital overlap concept ncert
Theoretical chemistry research group focusing on development of methods, and calculations in the areas of ionic liquids, photochemistry and catalysis
February 17, 2021 - We have all heard of the ozone layer depletion, haven’t we? Due to vast global warming and the rapid increase of temperature on earth, the ozone layer of the
Core orbitals are omitted. Marks 8 Using arrows to indicate electrons with their appropriate spin, indicate on the above diagram the ground state occupancy of the atomic orbitals of O and H, and of the molecular orbitals of OH. In the provided boxes on the above diagram, label the molecular orbitals as n, σ, σ∗, π, π∗, etc.
FREE Answer to 1) Prepare the molecular orbital diagram for the OH- ion
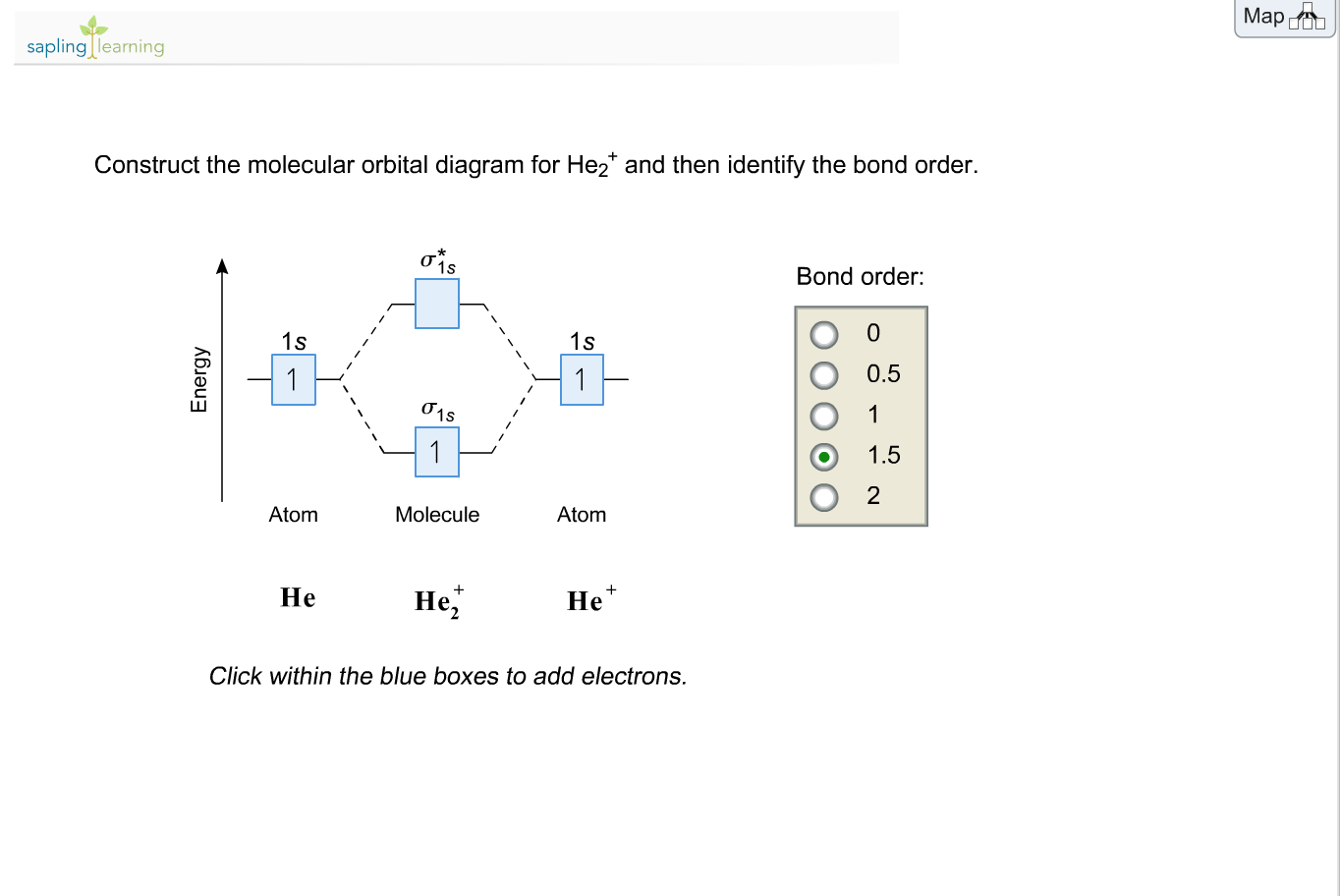
The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure On either side of the central ladder are shown the energies of the 1 s orbitals of atoms A and B, and the central two-rung ladder shows the energies of the bonding and antibonding.
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
d orbitals •l = 2, so there are 2l + 1 = 5 d-orbitals per shell, enough room for 10 electrons. •This is why there are 10 elements in each row of the d-block. σ‐MOs for Octahedral Complexes 1. Point group Oh 2. The six ligands can interact with the metal in a sigma or pi fashion.
• The energy increase of the e g orbitals and the energy decrease of the t 2g orbitals must be balancedrelative to the energy of the hypotheticalsphericalfield(aka the barycenter).• The energy of each of the two orbitals of the e g set rises by +3/5 o (+6 Dq) while the energy of eachof the three t 2g orbitalsfallsby ‐2/5 o(‐4Dq). • Thisresults inno netenergy changefor the system:
August 21, 2019 - The highest occupied molecular orbital (HOMO), 1b1, is predominantly pz2 in character with no contribution from the hydrogen 1s orbital and mainly contributes to the "lone pair" effects. The 2a1, 1b2 and 3a1 all contribute to the O-H bonds. The two lowest unoccupied molecular orbitals 4a1 (LUMO) ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. Compare the bond order in H 2 + and H 2 using the molecular orbital energy diagram for ...
In contrast to localizing electrons within their atomic orbitals in valence bond theory, the molecular orbital approach considers electrons to be delocalized across the entire molecule. The simple MO diagram of H 2O is shown on the right. Following simple symmetry treatments, the 1s orbitals ...
Molecular orbital diagram for the Fe3*Mn2*O,o clus-ter in the (a) ferromagnetic and (b) antiferromagnetic configu-rations. Orbitals indicated with a dashed line are unoccupied. Note that the orbital energies correspond to "orbital electronega-tivities" . Dec 05, · Upload failed.
Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals.
Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular…
#MOT #BMO #ABMO #HF #CO #NO #CN #OHHello everyoneThis is shivam here To follow me on instagram search - Sshivam898To join telegram group click on the given l...
1.The atomic orbital of the metal center and of surrounding ligands combine to form new orbitals, known as molecular orbitals. 2.The number of molecular orbitals formed is the same as that of the number of atomic orbitals combined. 3.The additive overlap results in the bonding molecular orbital while the subtractive overlap results in the
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Consider the hydroxide ion, OH and do the following: a. Prepare a molecular orbital diagram and fill with electrons given the following atomic orbital potential energies: O(2s) = -32.3 eV, O(2p) =-15.8 eV, H(1s) = -13.6 eV. Be sure to label each molecular orbital (bonding, anti-bonding, non-bonding). b. Sketch each molecular orbital's shape in ...



























0 Response to "44 oh- molecular orbital diagram"
Post a Comment