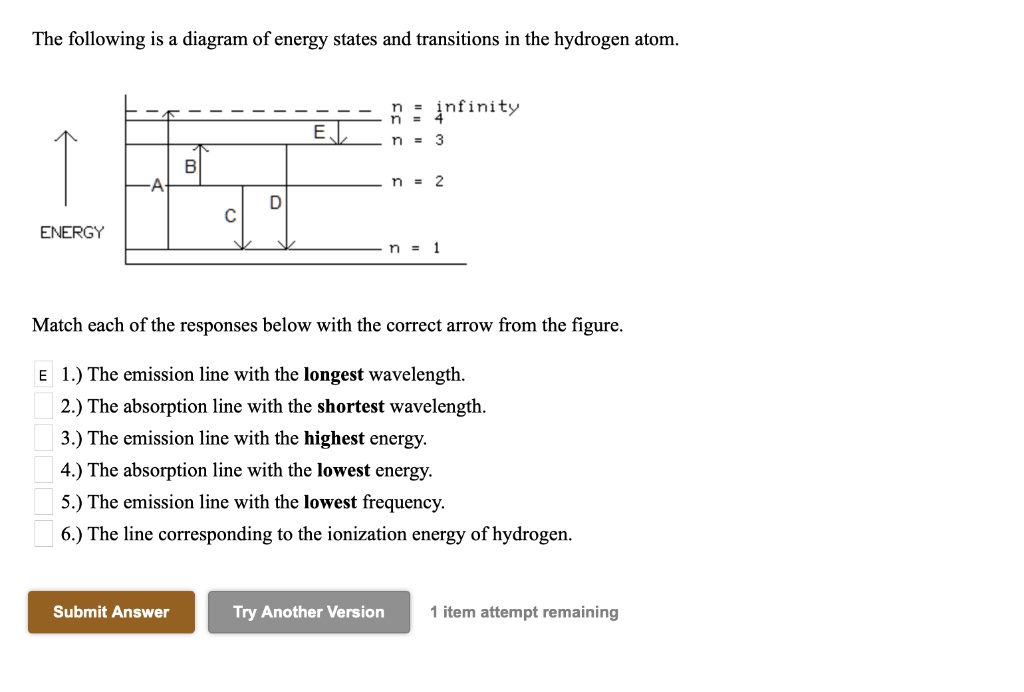
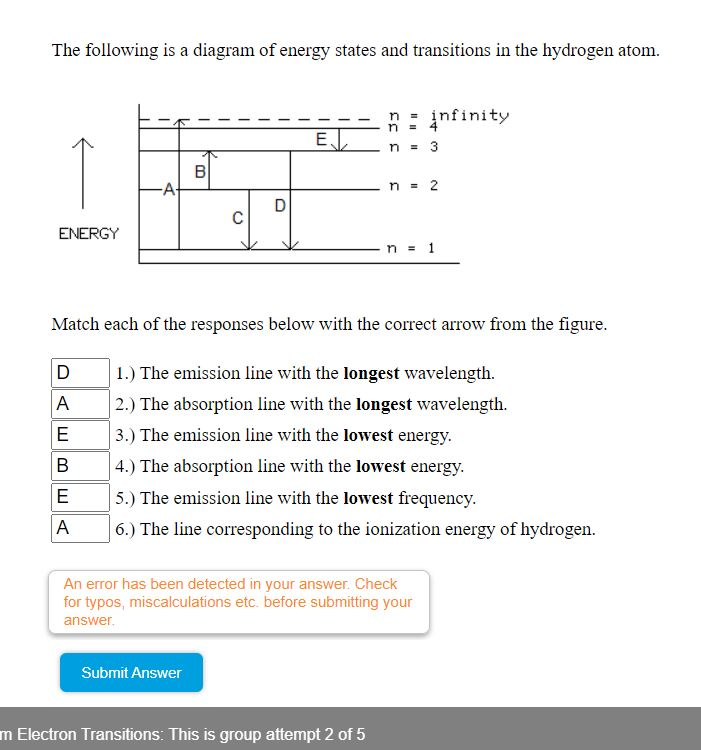
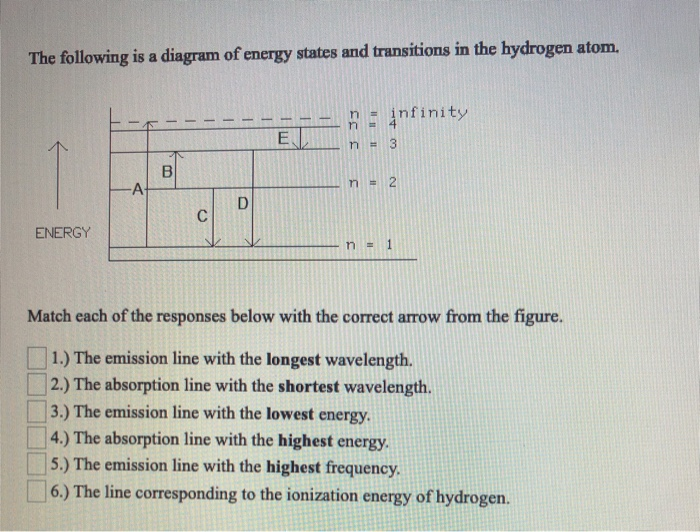
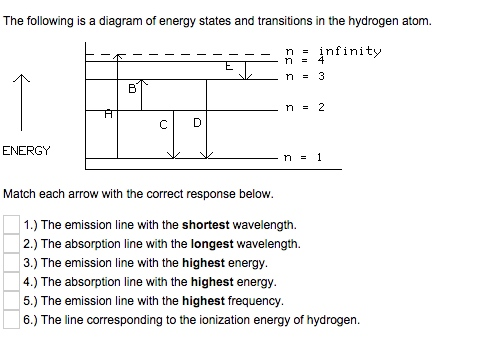
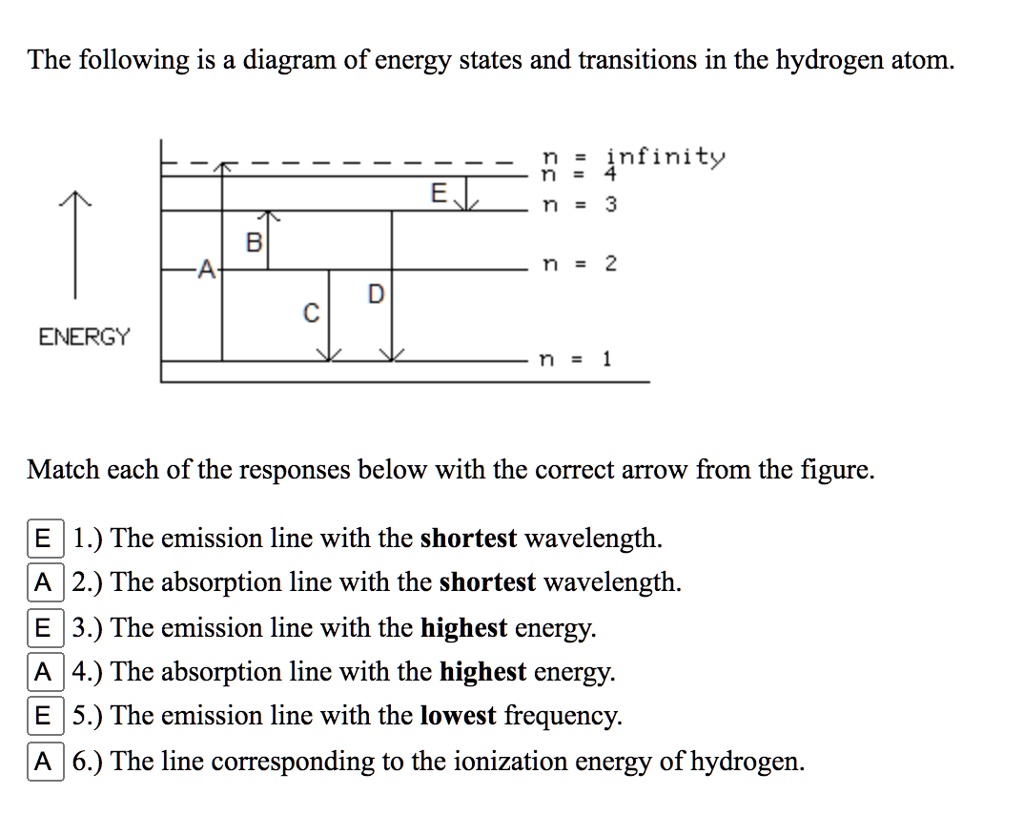
40 the following is a diagram of energy states and transitions in the hydrogen atom.
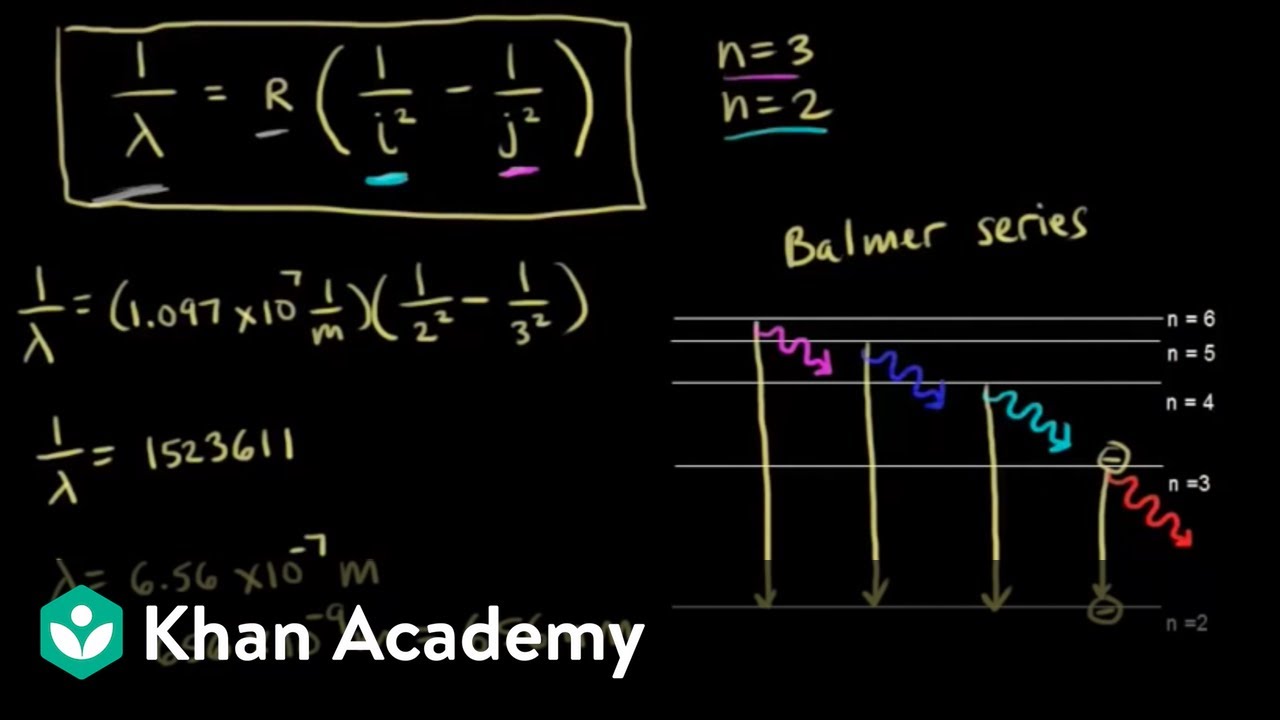
Energy of the Hydrogen-Like Atom. Remember Balmer series for hydrogen emission spectra obeys relation If stationary states in linear combination are degenerate, then linear combination is stationary state. Linear combinations of atomic orbitals are important in understanding covalent... The hydrogen atom consists of a nucleus which is just a single proton, and an electron encircling that nucleus. The energy levels of the electron determine the photons that the atom will absorb or emit, allowing the powerful scientific tool of spectral analysis.
Figure 5. An energy-level diagram plots energy vertically and is useful in visualizing the energy states of a system and the transitions between them. From Bohr's assumptions, we will now derive a number of important properties of the hydrogen atom from the classical physics we have covered in...
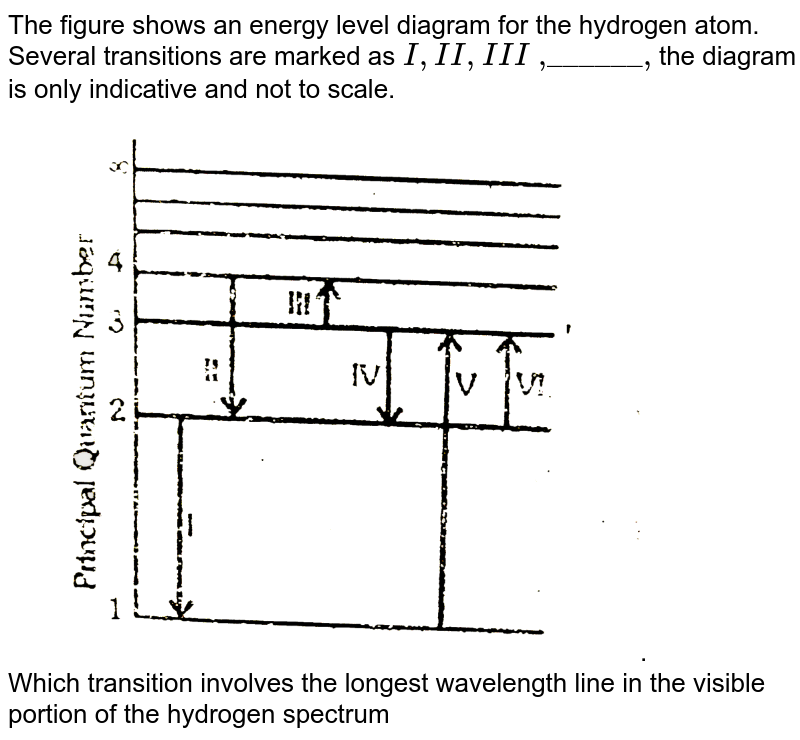
The following is a diagram of energy states and transitions in the hydrogen atom.
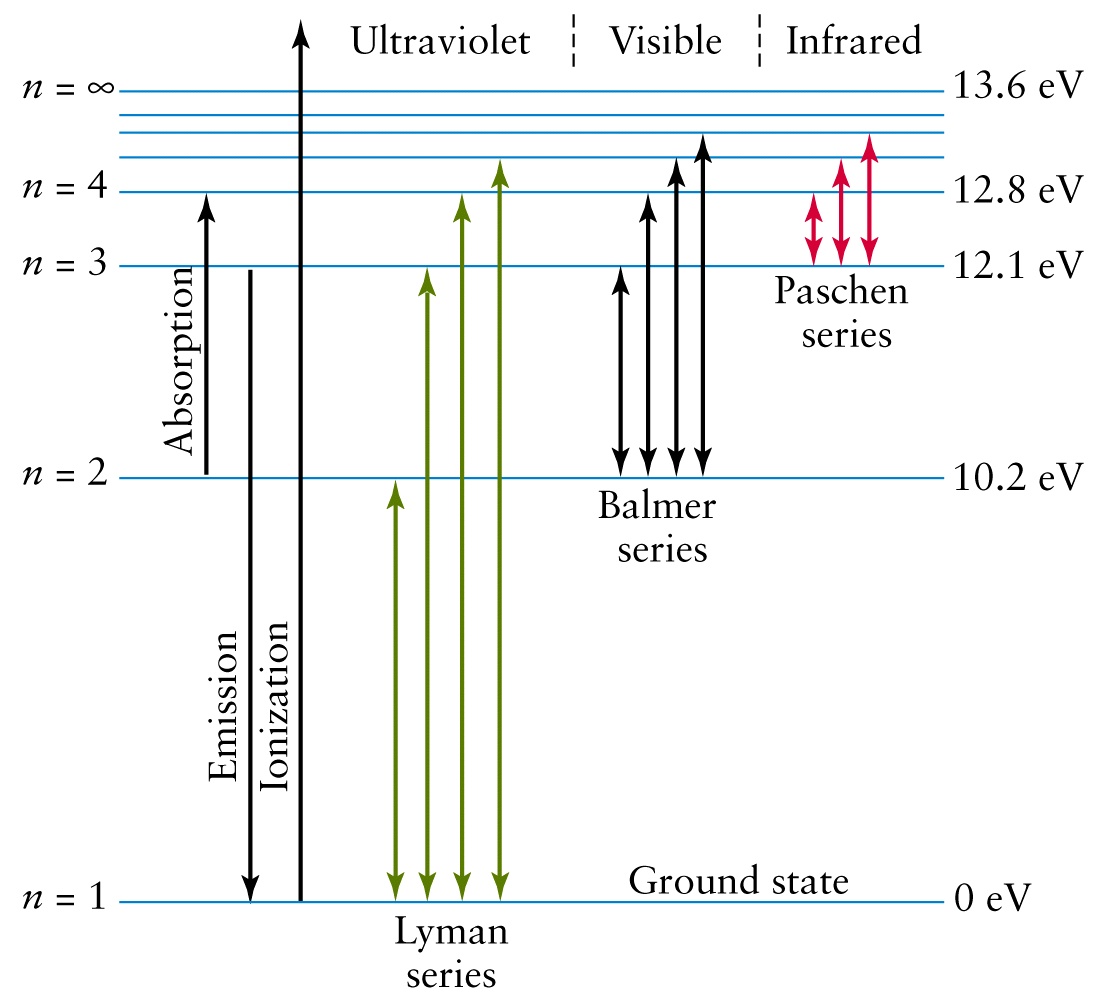
Problem: The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from the ...1 answer · Top answer: We are asked to match the responses with the correct arrow.Emission is a transition process from a higher energy level to a lower energy level. Absorption ... This diagram is for the hydrogen-atom electrons, showing a transition between two orbits We start by noting the centripetal force causing the electron to follow a circular path is supplied by the Coulomb force. Energy-level diagram for hydrogen showing the Lyman, Balmer, and Paschen series of... For the hydrogen atom, the energy levels only depend on the principal quantum number n. The energy levels are degenerate, meaning The lines for which nf = 1 are called the Lyman series. These transitions frequencies correspond to spectral lines in the ultraviolet region of the electromagnetic...
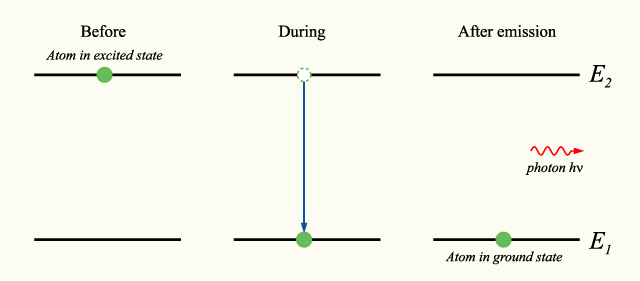
The following is a diagram of energy states and transitions in the hydrogen atom.. A schematic representation of magnetic energy states in the hydrogen atom. Electromagnetic radiation is emitted when the atom changes from the When two hydrogen atoms combine to form a hydrogen molecule, H2, they do so in a way quite different from the electron transfer process we... One transition between energy states of the hydrogen atom is represented by the picture on the left. 1. In this transition an electron moves from the n The size of the hydrogen atom will also reduce and the electron will move closer to the nucleus. This is because as energy increases, the electron is... Answer to: The following is a diagram of energy states and transitions in the hydrogen atom. By signing up, you'll get thousands of step-by-step...1 answer · Top answer: Emission spectra are observed when electrons return to the ground state from an excited state. Absorption spectra are observed when an electron goes ... Of the following transitions in the Bohr hydrogen atom, the ______ transition results in the emission of the lowest-energy photon.When the electron in a ...1 answer · 0 votes: Energy of transition between two levels = difference of energy in two levels = OE = hc = 'ho hi Planck's constant = 6.626 X 10-34 J. ci speed of light ...
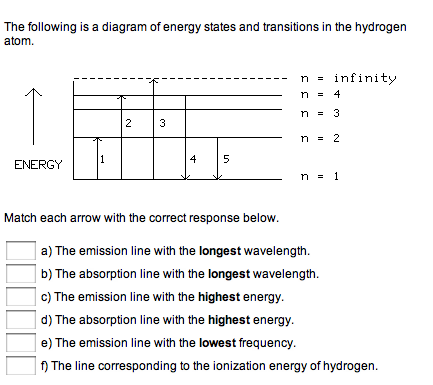
This chemistry video tutorial focuses on the bohr model of the hydrogen atom. It explains how to calculate the amount of electron transition energy that... Its energy levels are given in the diagram below. The x-axis shows the allowed energy levels of electrons in a hydrogen atom, numbered from 1 to 5 Electrons in a hydrogen atom must be in one of the allowed energy levels. If an electron is in the first energy level, it must have exactly -13.6 eV of... Spectroscopy of the Hydrogen Atom Transitions between the energy states (levels) of individual atoms give rise to characteristic atomic spectra. These spectra can be used as analytical tools to assess composition of matter. For instance, our knowledge of the atomic composition of the sun was... Transcribed image text: The following is a diagram of energy states and transitions in the hydrogen atom. Match each arrow with the correct response below. The ...1 answer · Top answer: wave length is inversely proportional to frequency of radiation the relation between frequency f and wavelength lamda f= hc/lamda Also energy and frequency ...
Transitions in Hydrogen. Let us calculate the rate of spontaneous emission between the first excited state (i.e., ) and the ground-state (i.e., ) of a hydrogen atom. Incidentally, since the state only has a finite life-time, it follows from the energy-time uncertainty relation that the energy of this state is... A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. The quantization of electron energy in the hydrogen atom is therefore. 6.5 Orbital Quantum Number: Designation of Angular-Momentum States. • This peculiar code originated in the empirical classification of spectra into series called sharp, principal, diffuse, and fundamental which occurred... Start studying Hydrogen Energy Levels. Learn vocabulary, terms and more with flashcards, games and other study tools. In the equation for energy of a hydrogen atom, E=-E0/n2, what is the Hopefully you have seen the lecture on atomic hydrogen transitions. If a higher-excited state electron drops...
The basic hydrogen energy level structure is in agreement with the Bohr model. If the radial probabilities for the states are used to make sure you understand the distributions of the probability The basic structure of the hydrogen energy levels can be calculated from the Schrodinger equation.
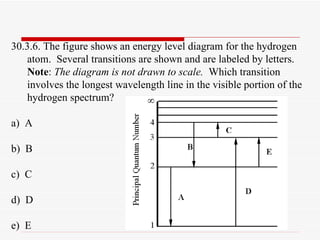
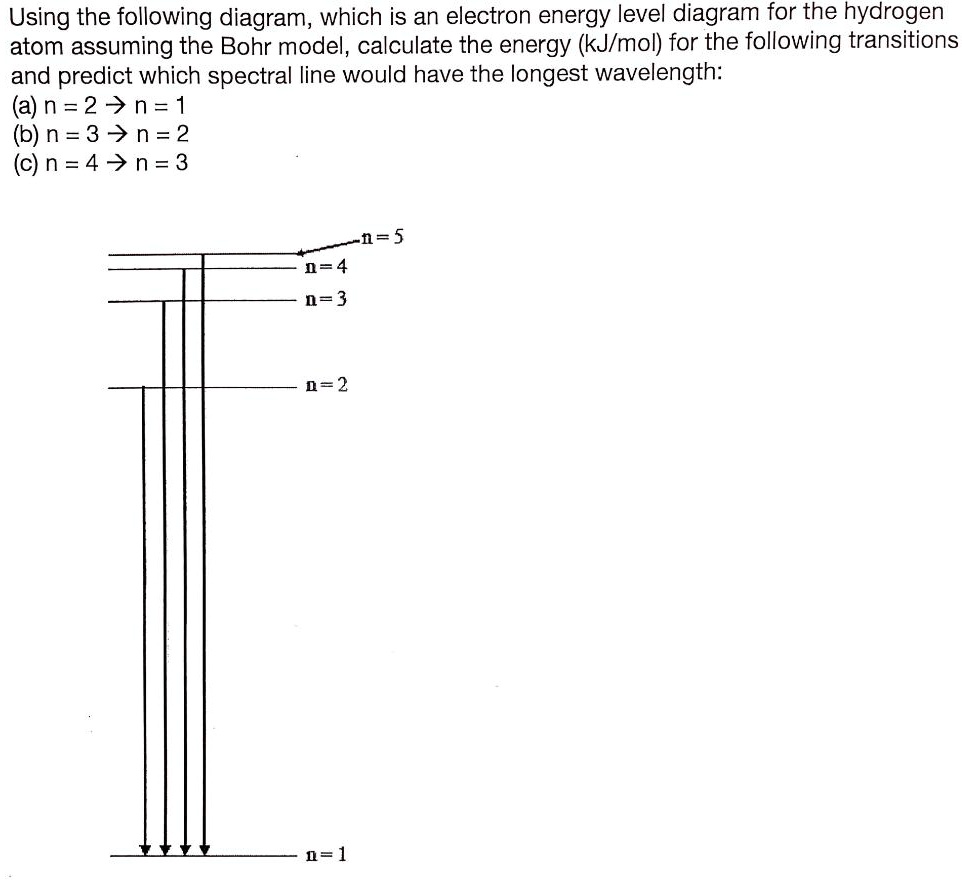
Draw the energy level diagram for the hydrogen atom and show transitions corresponding to lives of Lyman and Balmer series. > The energy required to excited an electron in hydrogen atom from the ground state to the next > When is Hα line in the emission spectrum of hydrogen atom obtained?
State one similarity and one difference in the observations you could make. (ii) Draw an arrow on the diagram to represent the lowest energy transition in the visible emission spectrum. (a) Complete the following table to show the numbers of sub-atomic particles in the species shown.
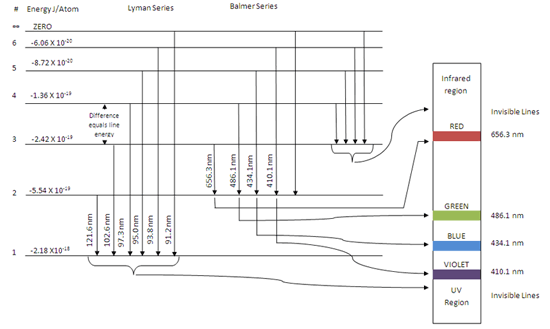
Atomic emission and absorption spectrum series, region and diagram for hydrogen atom, energy When this excited electron returns to the ground state it forms the following emission spectrum or The energy corresponding to a particular line in the emission and absorption spectra or spectrum of...
The Hydrogen Atom. is given at the bottom of the diagram. The two red vertical arrows are the first two transitions in the spectroscopic Balmer series, four lines of which gave Bohr the clue ground state are in the ultraviolet, they are called the Lyman series. Wave Functions for some Low-n States.
The energy level of the electron of a hydrogen atom is given by the following formula, where. It is quite obvious that an electron at ground state must gain energy in order to become excited. Since the energy level of the electron of a hydrogen atom is quantized instead of continuous, the spectrum...
drive energy transition across several 'hard-to-abate' sectors. However, investors should also be wary of Another episode of 'Hydrogen hype'? H2 revolution now appears credible with rapidly growth policy support Figure 6: Hydrogen atom is the lightest chemical element in the universe.
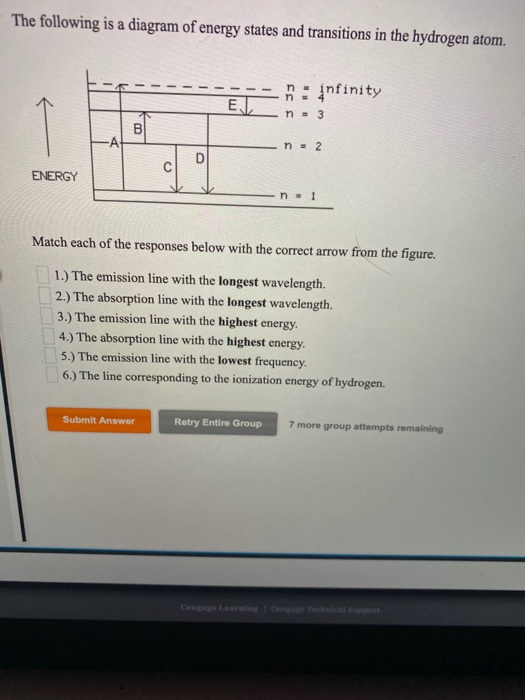
n. fullscreenExpand. Transcribed Image Text. The following is a diagram of energy states and transitions in the hydrogen atom. n = infinity n - 4 ...1 answer · Top answer: Step 1 Answer:-'This question is answered by using the simple concept of transition in hydrogen atom. Absorption involves the from lower to higher energy ...
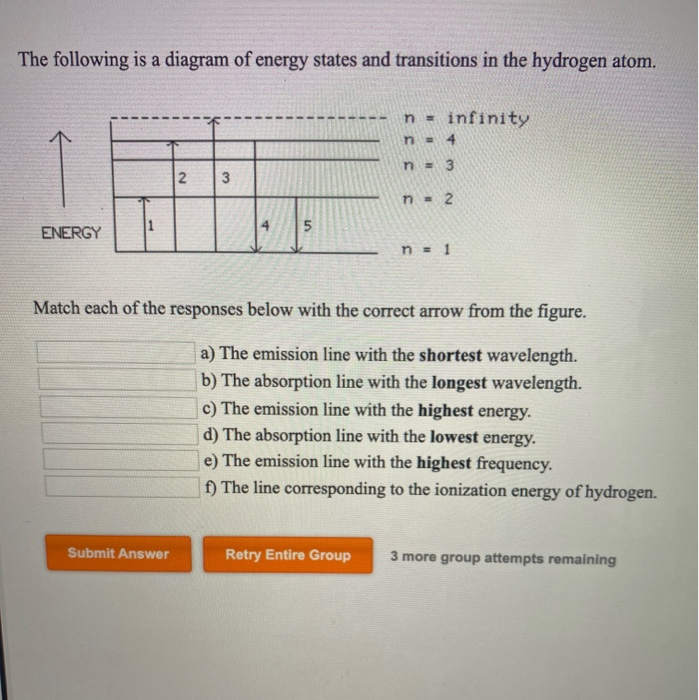
Match each arrow with the correct response below. The following diagram represents energy levels in a hydrogen atom.
Niels Bohr introduced the atomic Hydrogen model in 1913. These correspond to the emission of photons as an electron in an excited state transitions down to energy level n The Hydrogen atom can emit different wavelengths of light depending on the initial and final energy levels of the transition.
Orbital Energies and Atomic Structure. The energy of atomic orbitals increases as the principal Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom This is in accord with the Pauli exclusion principle: No two electrons in the same atom can have the...
The following is a diagram of energy states and transitions in the hydrogen atom: anfinity ENERGY Match each of the responses below with the correct arrow ...4 answers · Top answer: this problem. We are beginning to understand the electron transition that can occur in an atom. ...
Student has agreed that all tutoring, explanations, and answers provided by the tutor will be used to help in the learning process and in accordance with Studypool's honor code Let me know if you have questions. The following is a diagram of energy states and transitions in the hydrogen atom.
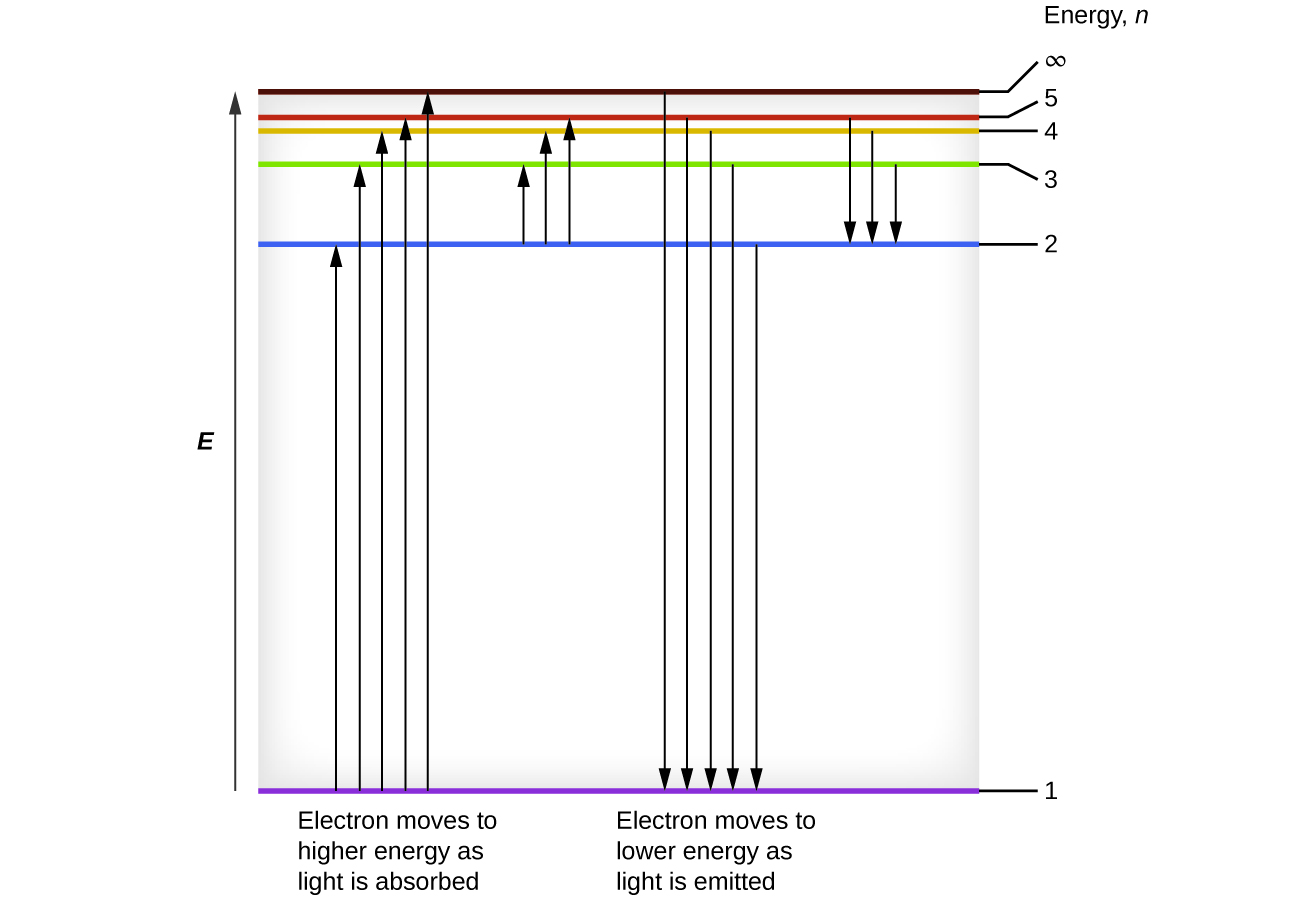
It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown If the electron in the atom makes a transition from a particular state to a lower state, it is losing energy. To conserve energy, a photon with an...
The energy levels of a hydrogen atom follow a regular pattern. We can depict an atomic transition graphically by drawing a little ball on the diagram to represent the energy of the atom. the hydrogen atom will absorb the photon and hop up to the n=2 level. That means that if we look at a...
Now in an hydrogen atom the probability of finding an electron in any non-stationary state is very very small. Hence an electron in this system is bound to be By joining hydrogen atoms (usually in the deuterium form, with one neutron) to make helium. This releases far more energy than any other...
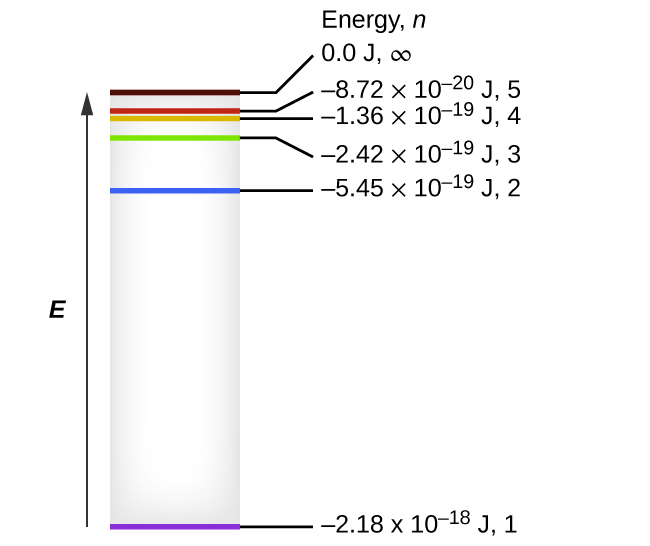
For any state corresponding to n in the hydrogen atom, you get E_n= -"13.6 eV"/n^2, where -"13.6 eV" is the approximate ground-state energy of the (Picture from Ohanian Physics). You can use the fact that a photon emitted during the transition from n = 5 to n = 2 will carry an energy #E# equal to...
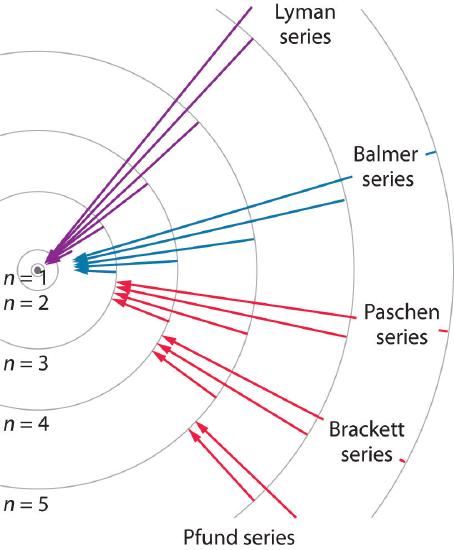
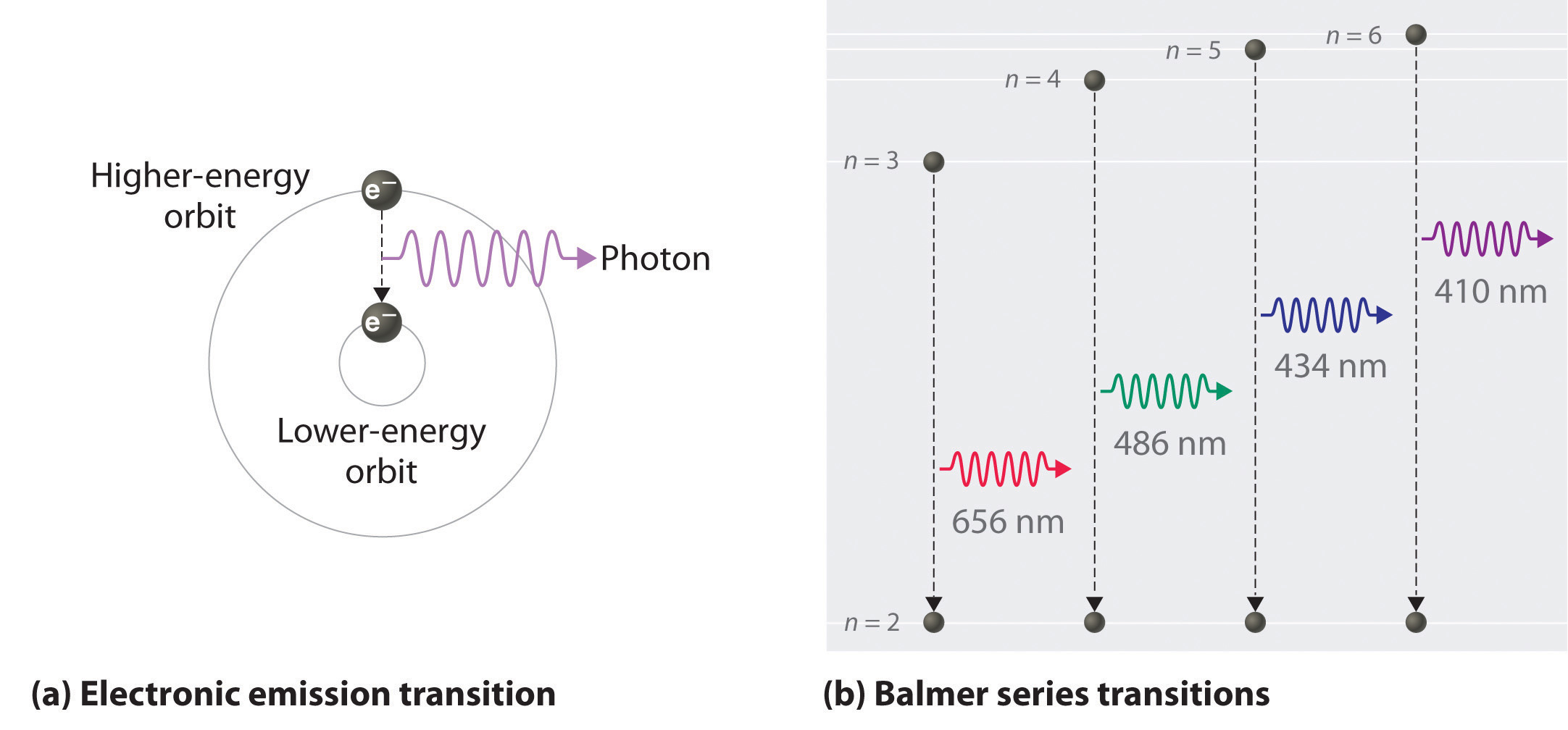
For the hydrogen atom, the energy levels only depend on the principal quantum number n. The energy levels are degenerate, meaning The lines for which nf = 1 are called the Lyman series. These transitions frequencies correspond to spectral lines in the ultraviolet region of the electromagnetic...
This diagram is for the hydrogen-atom electrons, showing a transition between two orbits We start by noting the centripetal force causing the electron to follow a circular path is supplied by the Coulomb force. Energy-level diagram for hydrogen showing the Lyman, Balmer, and Paschen series of...
Problem: The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from the ...1 answer · Top answer: We are asked to match the responses with the correct arrow.Emission is a transition process from a higher energy level to a lower energy level. Absorption ...













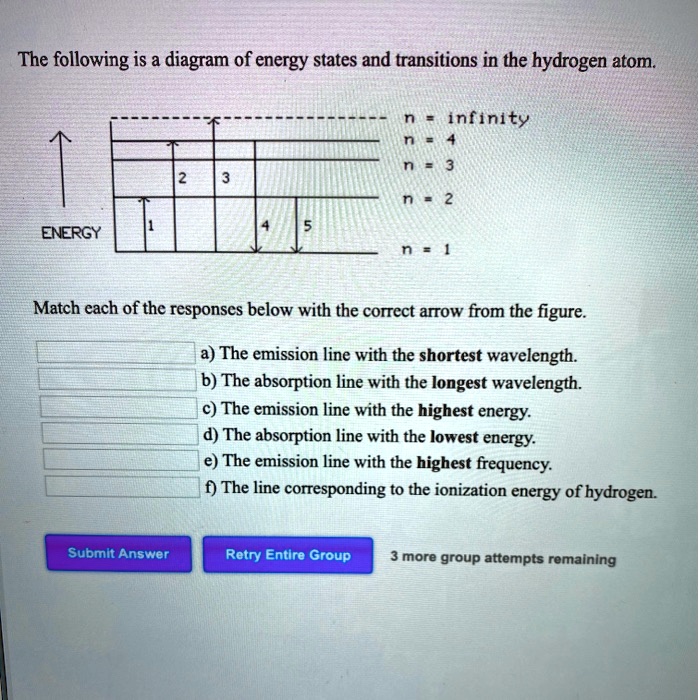
0 Response to "40 the following is a diagram of energy states and transitions in the hydrogen atom."
Post a Comment