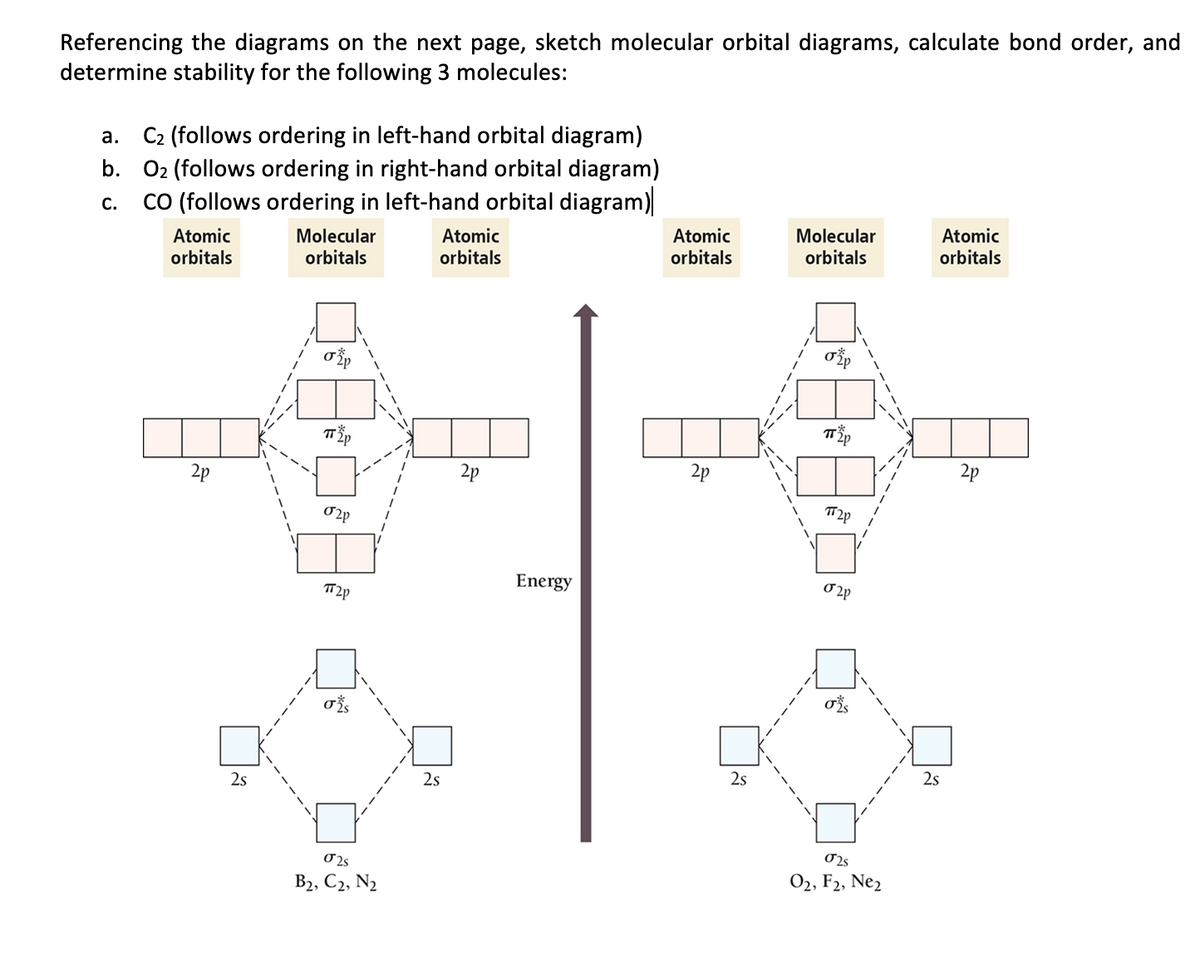
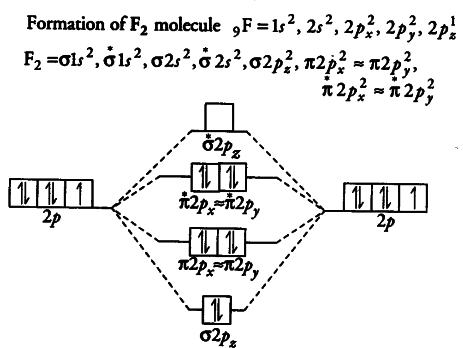
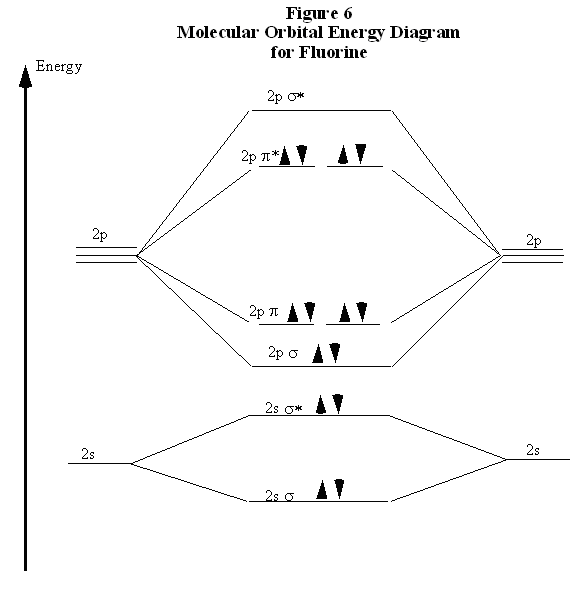
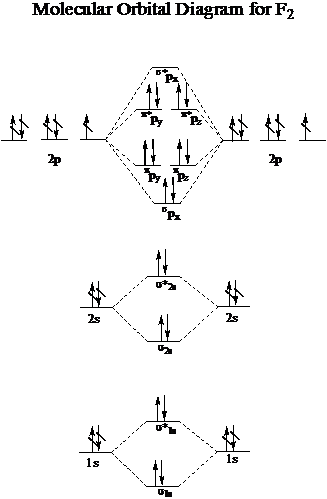
43 Molecular Orbital Diagram F2
Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons. F2 Molecular Orbital (MO) Diagram - Techiescientist As per molecular orbital (MO) theory, all the constituent atoms in a molecule contribute to the formation of molecular orbitals. Let us have a look at the MO diagram for F2. The 2s orbitals of both F atoms mix to form a low energy bonding orbital and a high energy antibonding orbital (as shown...
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... • Bonding - Review VSEPR and Hybridisation - Linear combination of molecular orbitals (LCAO), bonding / antibonding - Labelling of • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital Theory.
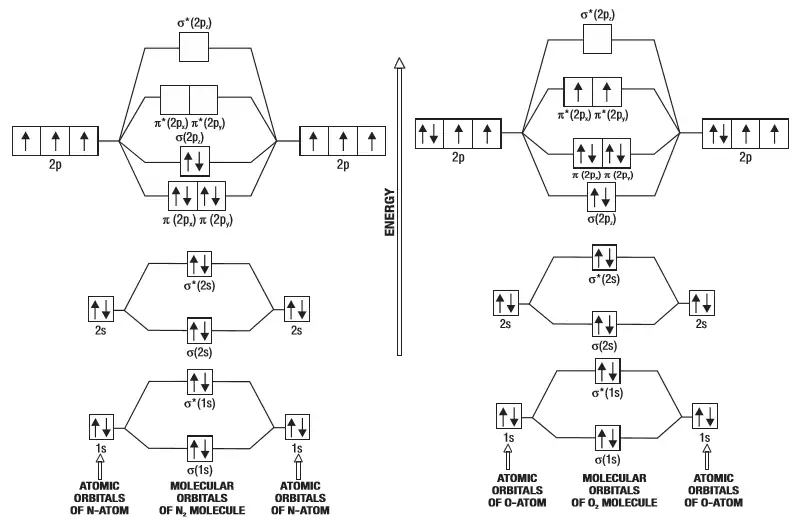
Molecular orbital diagram f2
Molecular Orbital Theory : chemhelp Molecular Orbital Theory. I'm having a lot of trouble with this stuff. I don't really know how to start these questions (such as how to draw So here, I basically ask, how do I draw a correlation diagram? Like, how do I know how many electrons to put in the bonding atomic orbital and antibonding atomic orbital? Molecular orbital diagram — Wikipedia Republished // WIKI 2 A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.[1][2][3] A fundamental principle of these theories is that... 8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals.
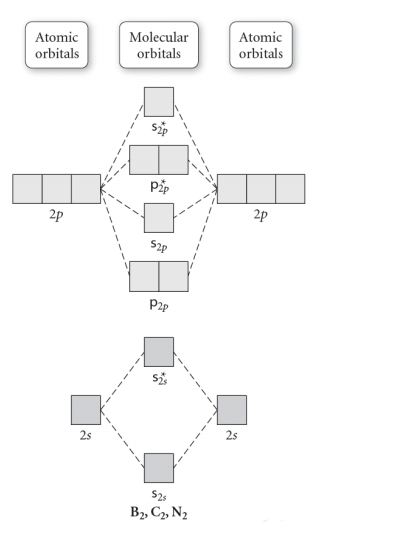
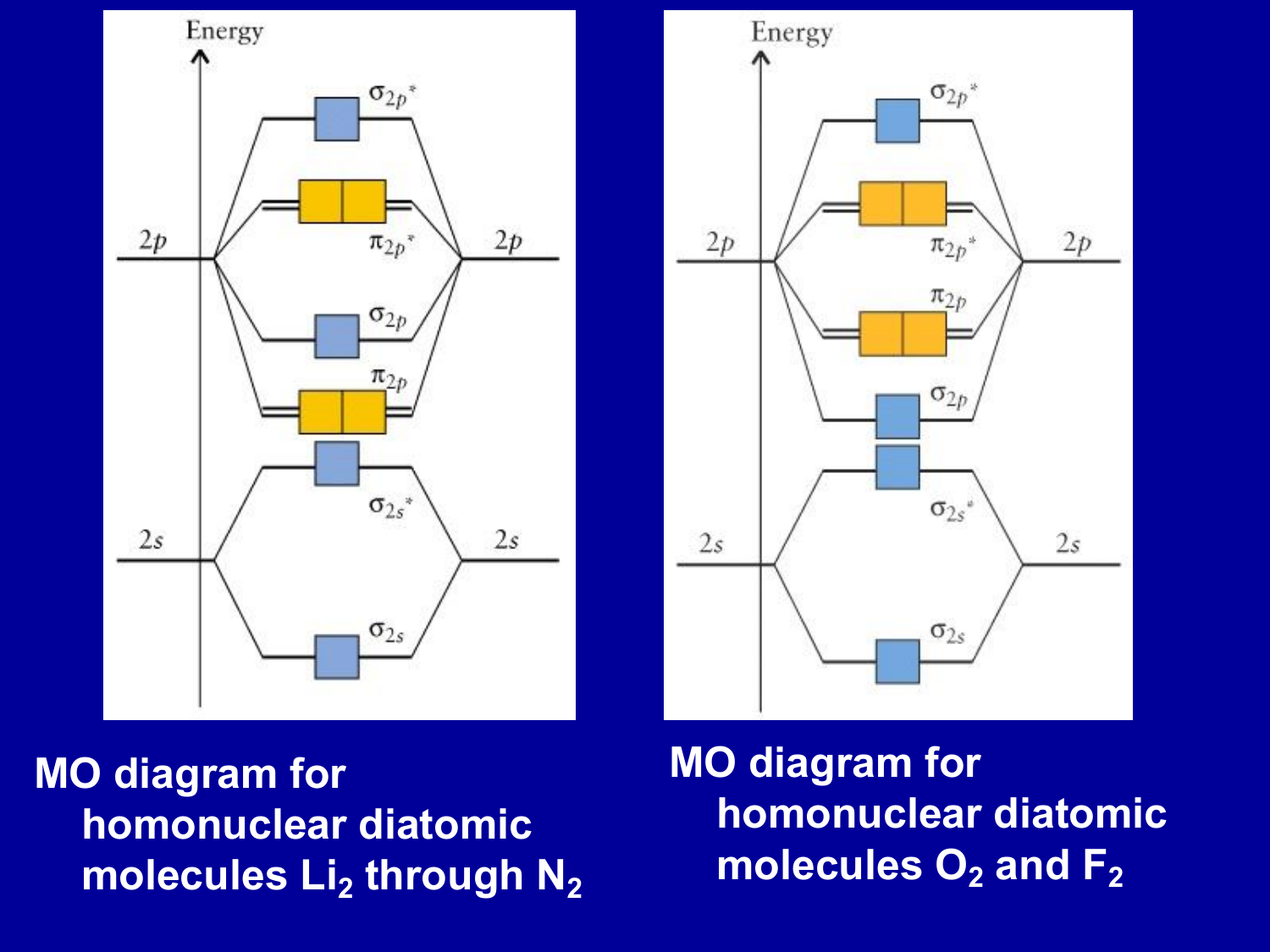
Molecular orbital diagram f2. CHAPTER 5: MOLECULAR ORBITALS bond order = 1 (like F2) Cl2 has the weakest bond. ... molecular orbitals in the diagram ... of valence electrons as F2, would have a single bond.29 pages MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row Molecular Orbital Theory - GeeksforGeeks The Molecular Orbital Theory is a chemical bonding theory developed at the turn of the twentieth century by F. R. Hund and R. S. Mulliken to explain the structure and properties of various molecules. The valence-bond theory failed to adequately explain how certain molecules, such as... Tutorial on Chemical Bonding, Part 8 of 10 (Molecular orbitals) The diagram shows how the molecular orbitals in lithium hydride can be related to the atomic orbitals of the parent atoms. Notice that the relative energies of the 2p-derived σ and π bonding molecular orbitals are reversed in O2 and F2. This is attributed to interactions between the 2s orbital each atom...
PDF Microsoft PowerPoint - Polyatomic Molecular Orbital Theory... Polyatomic Molecular Orbital Theory. Transformational properties of atomic orbitals. The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. Molecular Orbital Theory - BH3. The BH3 molecule exists in the gas phase, but... PDF Microsoft Word - Handin8s2017ans.docx 1. Sketch the qualitative molecular orbital diagram for XeF2. The molecule is linear and symmetric. The XeF2 is an electron excess molecule. You can think of the molecule as the complex between Xe and F2, both of which are closed shell. Energy level diagram for Molecular orbitals - Chemical Bonding and... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons. 3) If Nb = Na ,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of... [Expert Answer] Draw the molecular orbital diagram for... - Brainly.in Molecular orbital diagram and bond order of fluorine molecule. Fluorine molecule is formed by the combination of atomic orbitals of two fluorine atoms, each having nine electrons, thus making 18 electrons. These 18 electrons are filled in various molecular orbitals, in the increasing order of their...
Chapter 9 Molecular Orbitals in Chemical Bonding (Midterm) | Quizlet molecular orbital diagram for F2. number of elections in the pi*2p molecular orbital is. their molecular orbital diagrams are more symmetrical than those of homonuclear diatomic molecules. which of the following statements about nitrogen oxide, NO, is FALSE. Molecular Orbital Theory The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p Experiments have shown that O2 and F2 are best described by the model in the figure above, but B2, C2, and N2 are best... Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagrams are diagrams of MO energy levels, shown as short horizontal lines in the center. Atomic orbitals (AO) energy levels are shown for comparison. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels. What is the molecular orbital diagram for B_2? | Socratic Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals.
What is the molecular orbital diagram of O2 and F2? - Quora Something went wrong. Wait a moment and try again. Try again.
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
8.4 Molecular Orbital Theory - Chemistry Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. Figure 8. This is the molecular orbital diagram for the homonuclear diatomic Be2+, showing the molecular orbitals of the valence shell only.
PDF Figure 9.32: The molecular orbital energy-level diagram for • The following slide illustrates the relative energies of the molecular orbitals compared to the original atomic orbitals. • Because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable. Figure 9.26: (a) The molecular orbital energy-level diagram for the...
PDF Chapter 5 | 5.2.2 Orbital Mixing Molecular orbital theory uses group theory to describe the bonding in molecules; it comple-ments and extends the introductory bonding models in Chapter 3 . In molecular orbital theory the symmetry properties and relative energies of atomic orbitals determine how these orbitals interact to form...
PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams Figure 9-2 Molecular orbital (MO) diagram for the combination of the 1s atomic orbitals on two identical atoms (at the left) to form two MOs. Molecular orbital calculations indicate, however, that for O2, F2, and hypothetical Ne2 molecules, the 2p orbital is lower in energy than the 2p orbitals...
Molecular Orbital Theory (MOT), Chemistry Study... | eMedicalPrep The molecular orbital diagram representing this order of energy levels is shown in fig. This kind of mixing of orbitals or symmetry interaction is not applicable for O2 and F2 molecule formation because of larger energy gap between 2s and 2p orbitals for these atoms.
Figure 2. Qualitative molecular orbital diagram of HF −. The 2σ... Download scientific diagram | Qualitative molecular orbital diagram of HF −. The 3σ orbital is a combination of the F 2p z and H 1 s orbitals and is bonding, whereas the F 2p x and 2p y orbitals cannot interact with the H 1 s orbital due to different symmetries and serve as non-bonding orbitals.
Asked for: "skewed" molecular orbital energy-level diagram, bonding... Figure 4.10.1: Molecular Orbital Energy-Level Diagrams for Homonuclear Diatomic Molecules.(a) For F2, with 14 valence electrons (7 from each To obtain the molecular orbital energy-level diagram for O2, we need to place 12 valence electrons (6 from each O atom) in the energy-level diagram shown...
Molecular Orbital (MO) Diagram for F2(2+) - YouTube When two fluorine atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.F2(2+) has a bond order of 2, so...
What is the molecular orbital diagram of O2 and F2? - Quora Both oxygen and fluorine have the same MO diagram with the difference only in the occupation of orbitals. Image courtesy Singlet oxygen - Wikipedia.6 answers · 61 votes: Here is the solution, %3E * For O2 molecule, %3E * For F2 molecule, Thanks for reading.
Molecular Orbital Diagrams simplified | by Megan A. Lim | Medium Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory. Valence Bond Theory proposes that electrons are localized between two atoms.
Molecular orbital diagram for BF3 - Chemistry Stack Exchange I'm trying to build a molecular orbital diagram for BF3 and I'm running into problems with irreducible representations on the F side. I found the following diagram for BF3 online but it doesn't generate the E' anti bonding and also doesn't generate enough molecular orbitals.
8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals.
Molecular orbital diagram — Wikipedia Republished // WIKI 2 A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.[1][2][3] A fundamental principle of these theories is that...
Molecular Orbital Theory : chemhelp Molecular Orbital Theory. I'm having a lot of trouble with this stuff. I don't really know how to start these questions (such as how to draw So here, I basically ask, how do I draw a correlation diagram? Like, how do I know how many electrons to put in the bonding atomic orbital and antibonding atomic orbital?




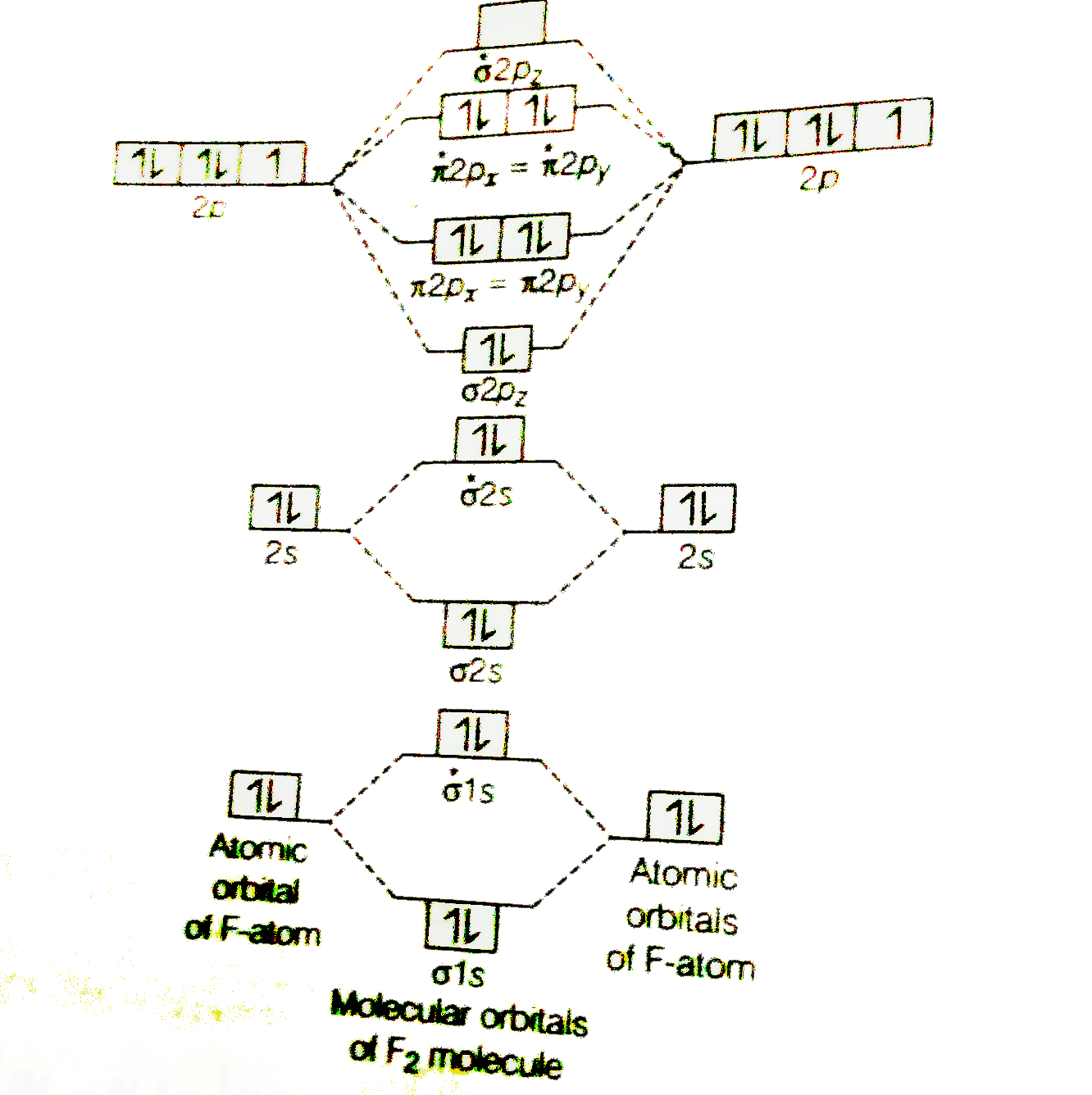

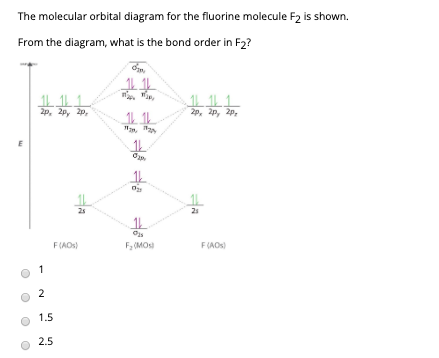
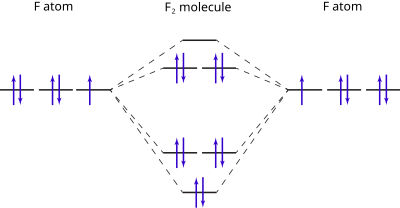




![Expert Answer] Draw the molecular orbital diagram for F2 and ...](https://hi-static.z-dn.net/files/dae/d7baa23a1d4a2ea2c90e0a703e2fd41d.jpg)







0 Response to "43 Molecular Orbital Diagram F2"
Post a Comment