43 orbital diagram of as
How to Draw Orbital Diagrams - YouTube Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau... 7.7 Molecular Orbital Theory - Chemistry Fundamentals Molecular Orbital Energy Diagrams. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right.
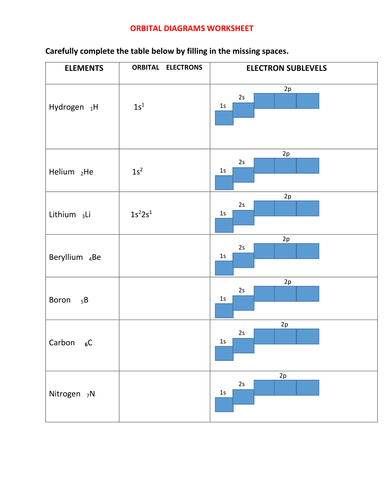
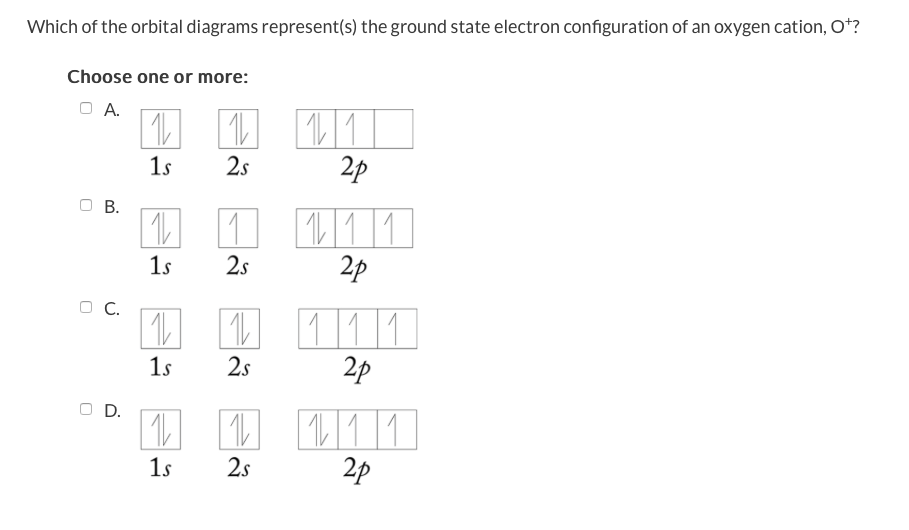
Orbital Diagrams Chemistry Tutorial - AUS-e-TUTE An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s.

Orbital diagram of as
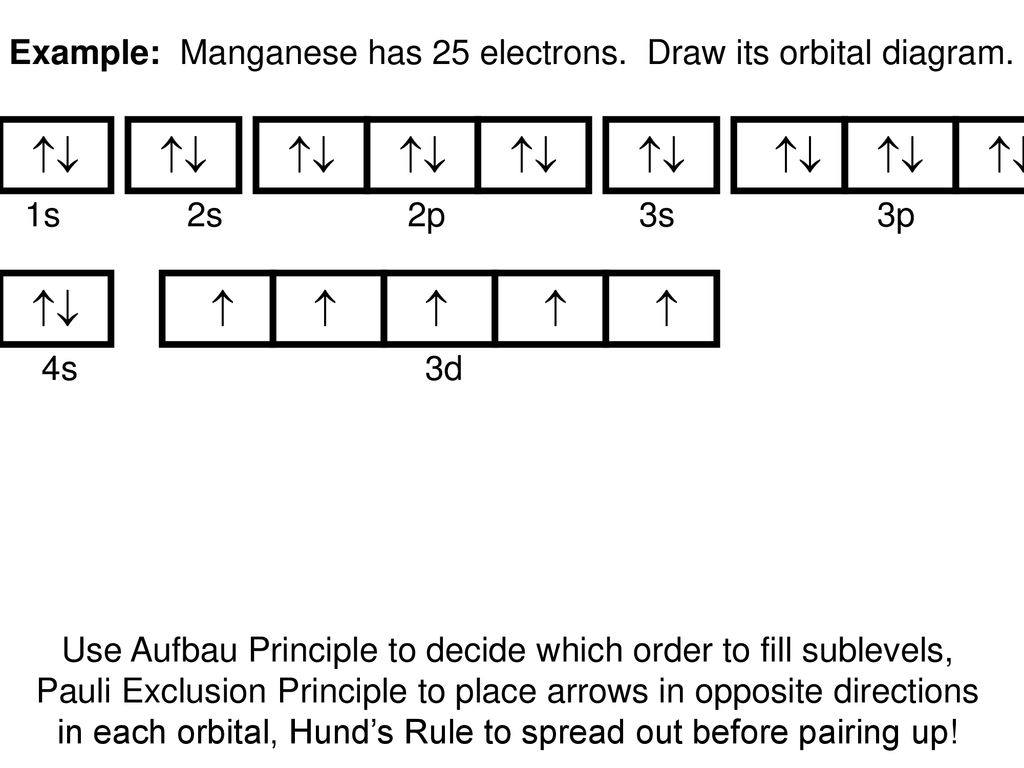
PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom Orbital Diagrams and Electron Configuration - Basic ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... ssd.jpl.nasa.gov › tools › sbdb_lookupSmall-Body Database Lookup - NASA Instructions. The search form recognizes IAU numbers, designations, names, and JPL SPK-ID numbers. When searching for a particular asteroid or comet, it is best to use either the IAU number, as in 433 for asteroid “433 Eros”, or the primary designation as in 1998 SF36 for asteroid “25143 (1998 SF36)”.
Orbital diagram of as. Carbon Orbital diagram, Electron configuration, and ... Orbital diagram:-A orbital diagram is simply a pictorial representation of the arrangement of electrons in the orbital of an atom, it shows the electrons in the form of arrows, also, indicates the spin of electrons.Electron configuration:- Electron configuration is the arrangement of electrons in atomic orbitals.It shows the electrons in numbers, It doesn't show the details on the spin of ... valenceelectrons.com › oxygen-electron-configurationOxygen(O) electron configuration and orbital diagram Oxygen(O) is the 8th element in the periodic table and its symbol is ‘O’. This article gives an idea about the electron configuration of oxygen and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles. Orbital Diagram For Carbon (C) | Carbon Electron Configuration Carbon Electron Dot Diagram. If we talked about the electronic configuration of the element then, carbon is an element whose electronic configuration is given as 1s22s22p2. Now the main thing here is what electronic configuration means, so the solution/ answer is that in simple words, by knowing the electronic configuration of any element we ... Orbital Filling Diagram For Sulfur The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows. Energy levels: 2, 8, 6 Orbitals: 1s2 2s2 2p6 3s2 3p4 If you need to fill in the little boxes, here's one for you. Each arrow represents one electron.
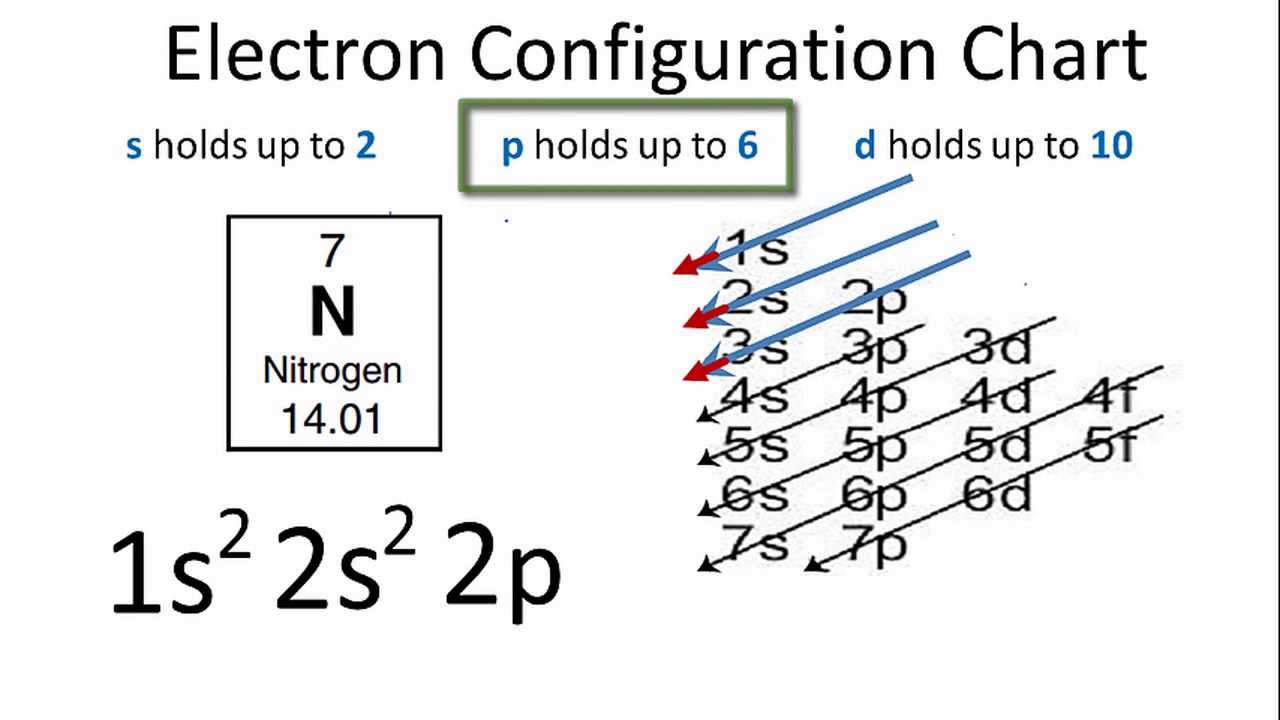
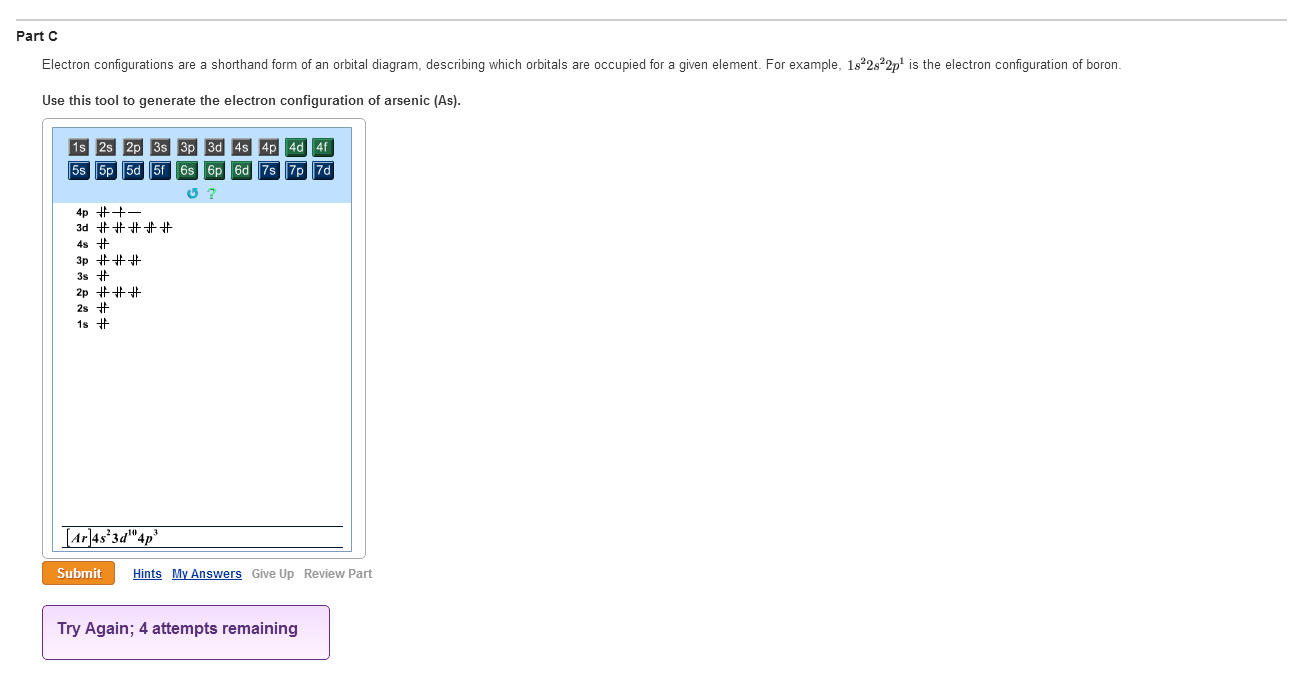
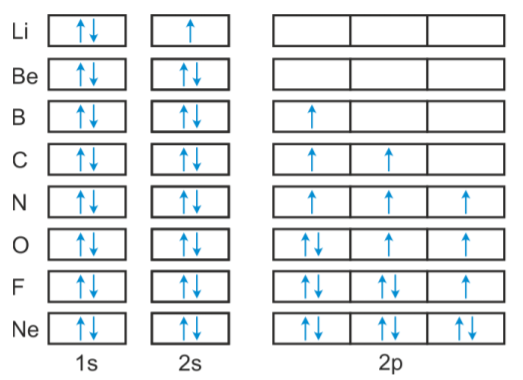
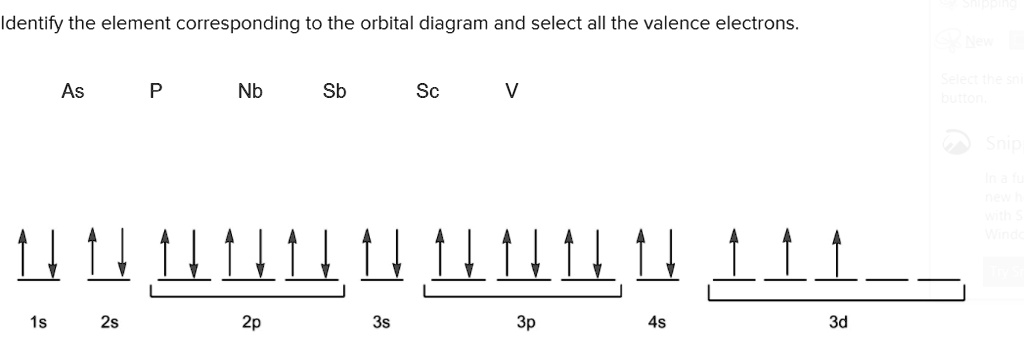
Orbital Diagram For Arsenic - schematron.org The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org! Arsenic atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. topblogtenz.com › cyanide-cn-lewis-structureCN- lewis structure, molecular orbital diagram, and, bond order Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 2. Find if the molecule homo-nuclear diatomic molecular orbital or hetero-nuclear diatomic molecular orbital. Clearly, CN is hetero orbital. 3. Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of: › question-answer › draw-theDraw the molecular orbital diagram of N2N2 + N2 Write class ... Molecular orbital diagram explains the chemical bonding in the molecules in terms of molecular orbital theory. In a molecular orbital diagram, the diagram of molecular orbital energy levels is shown as horizontal lines. Degenerate orbitals (orbitals having the same energy) are shown side by side in these diagrams.
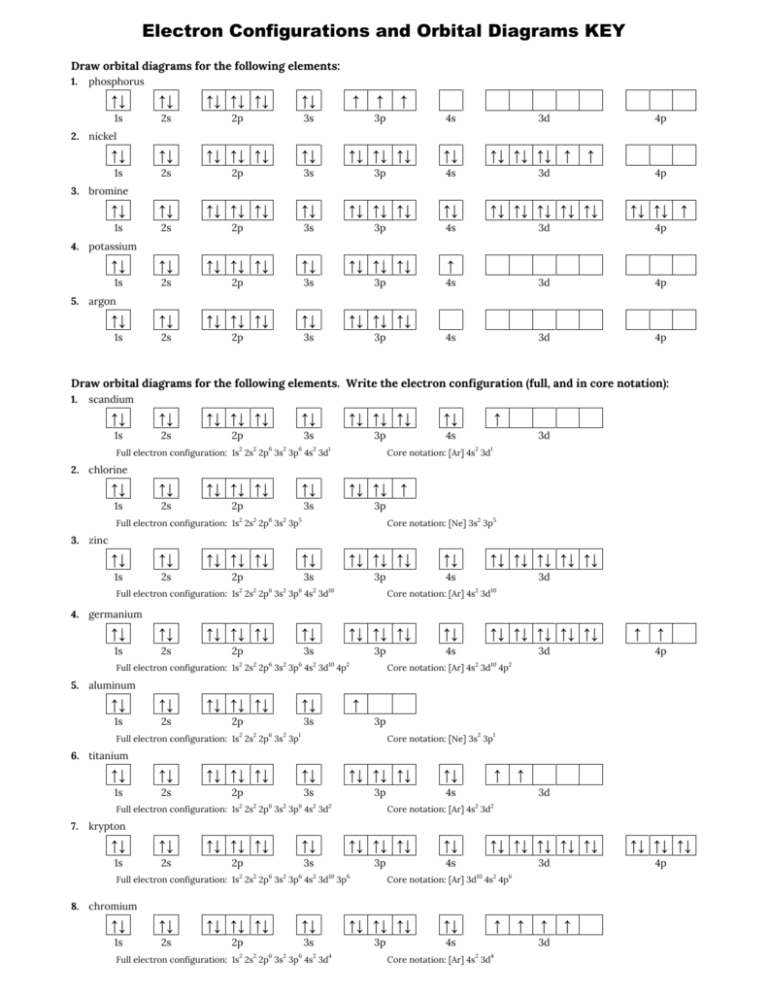
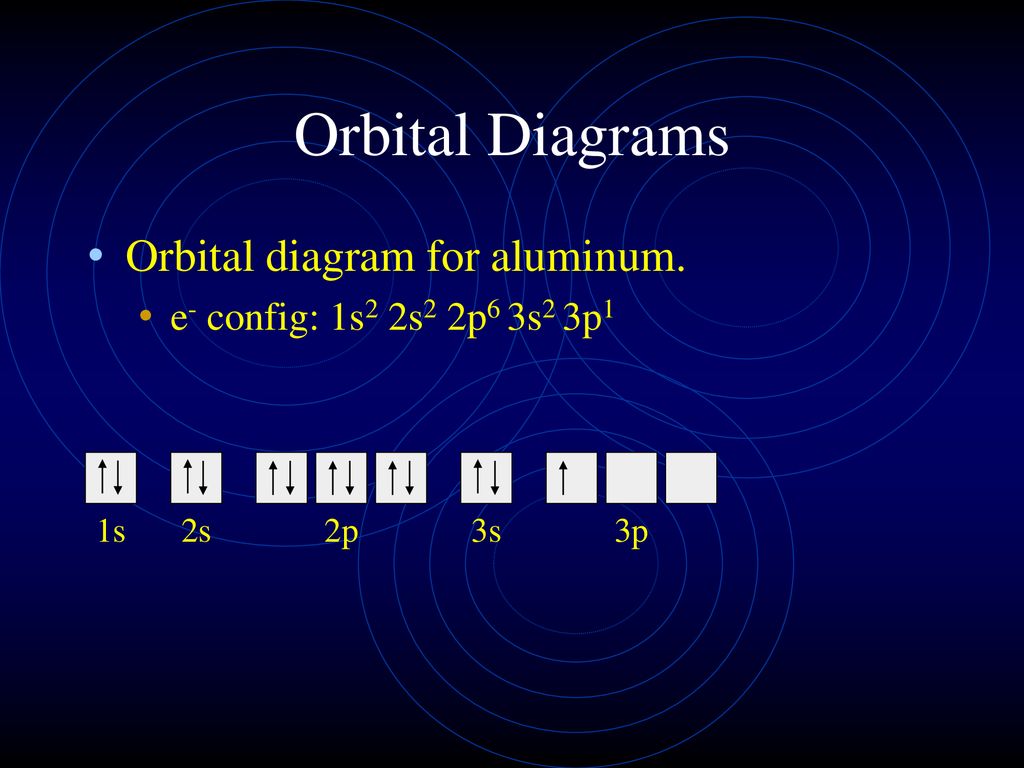
Silicon Electron Configuration | Orbital Diagram For ... Silicon Orbital Diagram. Well, the orbital diagram of Electron Configuration is the best source of information if you are willing to study the chemical bonding of silicon. This is a kind of qualitative diagram that explains the whole combining process of the element in the best possible manner. PDF Electron Configurations and Orbital Diagrams key Draw orbital diagrams for the following elements. Write the electron configuration (full, and in core notation): 1. scandium ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑ 1s 2s 2p 3s 3p 4s 3d Full electron configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1 Core notation: [Ar] 4s 2 3d 1 2. Nitrogen Orbital diagram, Electron configuration, and ... Orbital diagram:-A orbital diagram is simply a pictorial representation of the arrangement of electrons in the orbital of an atom, it shows the electrons in the form of arrows, also, indicates the spin of electrons.Electron configuration:- Electron configuration is the arrangement of electrons in atomic orbitals.It shows the electrons in numbers, It doesn't show the details on the spin of ... Aluminum(Al) electron configuration with orbital diagram Aluminum (Al) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. The first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. The 1s orbital is now filled with two electrons.
What is molecular orbital diagram of CO? - handlebar ... What is an orbital energy diagram? Orbital diagrams are a pictorial description of electrons in an atom. In order to figure out where electrons go in an atom we have to follow 3 main rules. The first one being the Auf Bau Principle, the Auf Bau Principle states that each electron occupies the lowest energy orbital available.
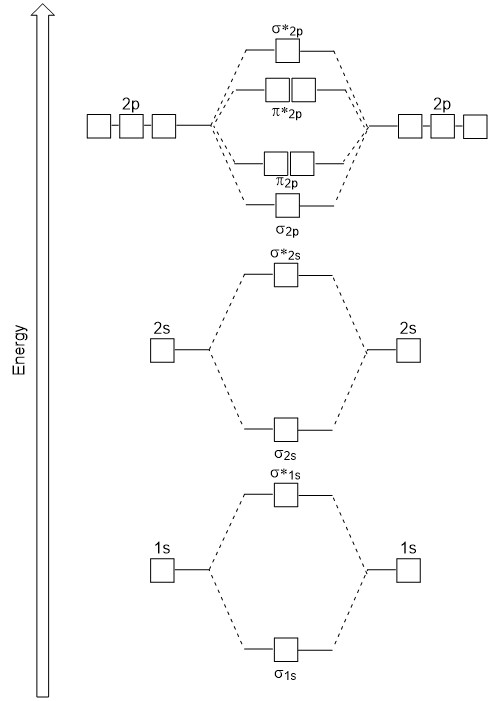
en.wikipedia.org › wiki › Molecular_orbital_diagramMolecular orbital diagram - Wikipedia Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
Beryllium Orbital Diagram - schematron.org Beryllium Orbital Diagram. A quiz solution for Inorganic Chemistry in which students were prompted to draw the molecular orbital diagram for beryllium hydride. Beryllium is the fourth element with a total of 4 electrons. In writing the electron configuration for beryllium the first two electrons will go in the 1s orbital.
What is the electron configuration, orbital diagram, and ... So the electron configuration of potassium will involve 19 electrons. The full electron configuration of potassium is "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"4s"^1". The noble gas notation is "[Ar]4s"^1". The following orbital diagram shows the increase in energy from one energy sublevel to the next, but you can write them on the same level horizontally,
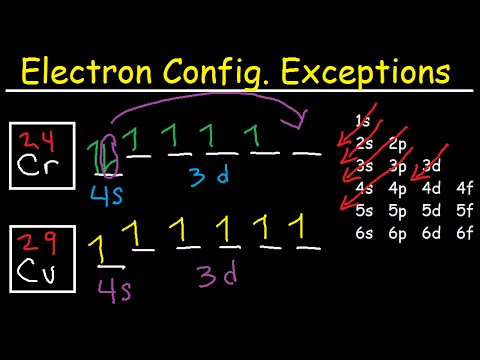
Bromine Orbital Diagram - Wiring Diagrams Bromine Orbital Diagram. Answer to Write the electron configuration and give the orbital diagram of a bromine (Br) atom (Z = 35). the σ bonds. I've drawn the overlaps below in the MO diagrams. Each bromine would donate one 4pz electron to form a σ -bonding orbital. Answer to Draw an orbital diagram for each element: (a) magnesium; (b ...
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Orbital Diagram For Beryllium The MO diagram is the diagram as a whole. A molecular orbital diagram, Beryllium has an electron configuration 1s 2 2s 2, so there are again two electrons in the valence level. However, the 2s can mix with the 2p orbitals in diberyllium, whereas there are no p orbitals in the valence level of hydrogen or helium. The orbital filling diagram of ...
PDF The Electronic Structure of Ferrocene A qualitative molecular orbital diagram for ferrocene (D 5d) FeII Fe SALC's p p a 2u * e 1u * x e 1u z y a e 2g * e 2g, u a 2u, e 1u a 1g * u a 1g e 1g * LUMO a 1g, e 1g, e 2g e 2g a 1g HOMO dyz dxz e 1g e 1g e 1u e 1g, e 1u a 1g a 2u a 1g, a 2u
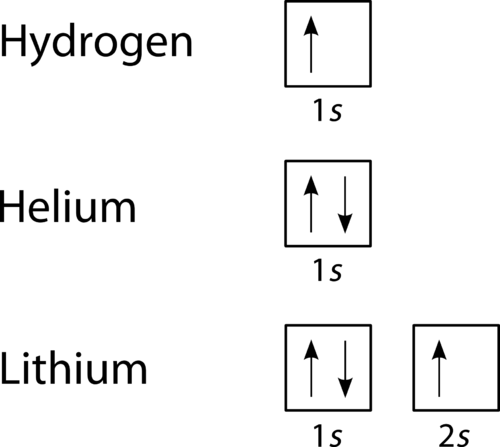
How do you write the orbital diagram for carbon? | Socratic The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is #2# , each with opposite spins (Pauli's exclusion principle). In a neutral carbon atom, the #"1s"# sublevel has one orbital with two electrons with opposite spins, represented by the arrows pointing in opposite ...
Molecular Orbital Theory - Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution We draw a molecular orbital energy diagram similar to that shown in .
8 - Drawing Molecular Orbital Diagrams — Flux Science 8 - Drawing Molecular Orbital Diagrams. Abstract (TL;DR) Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds. Depending on if it is a homonuclear case, where the bonding atoms are the same, or a heteronuclear case, where the bonding atoms are ...
Orbitals Chemistry (Shapes of Atomic Orbitals) - Shape of ... The orbital wave function or ϕ is a mathematical function used for representing the coordinates of an electron. The square of the orbital wave function or represents the probability of finding an electron. This wave function also helps us in drawing boundary surface diagrams.
valenceelectrons.com › neon-electron-configurationNeon(Ne) electron configuration and orbital diagram To write the orbital diagram of neon(Ne), you have to do the electron configuration of neon. Which has been discussed in detail above. Neon (Ne) orbital diagram. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in ...
Orbital Diagrams - Concept - Chemistry Video by Brightstorm Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. The Pauli Exclusion Principle says that only two electrons can fit into an single orbital.
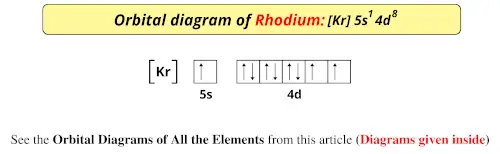
periodictableguide.com › orbital-diagram-of-allOrbital Diagram of All Elements (Diagrams given Inside) Apr 10, 2021 · Orbital Diagram of All Elements (Diagrams given Below) January 1, 2022 April 10, 2021 by Admin Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below.
Sulfur Orbital diagram, Electron configuration, and ... Orbital diagram:-A orbital diagram is simply a pictorial representation of the arrangement of electrons in the orbital of an atom, it shows the electrons in the form of arrows, also, indicates the spin of electrons.Electron configuration:- Electron configuration is the arrangement of electrons in atomic orbitals.It shows the electrons in numbers, It doesn't show the details on the spin of ...
ssd.jpl.nasa.gov › tools › sbdb_lookupSmall-Body Database Lookup - NASA Instructions. The search form recognizes IAU numbers, designations, names, and JPL SPK-ID numbers. When searching for a particular asteroid or comet, it is best to use either the IAU number, as in 433 for asteroid “433 Eros”, or the primary designation as in 1998 SF36 for asteroid “25143 (1998 SF36)”.
Orbital Diagrams and Electron Configuration - Basic ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom












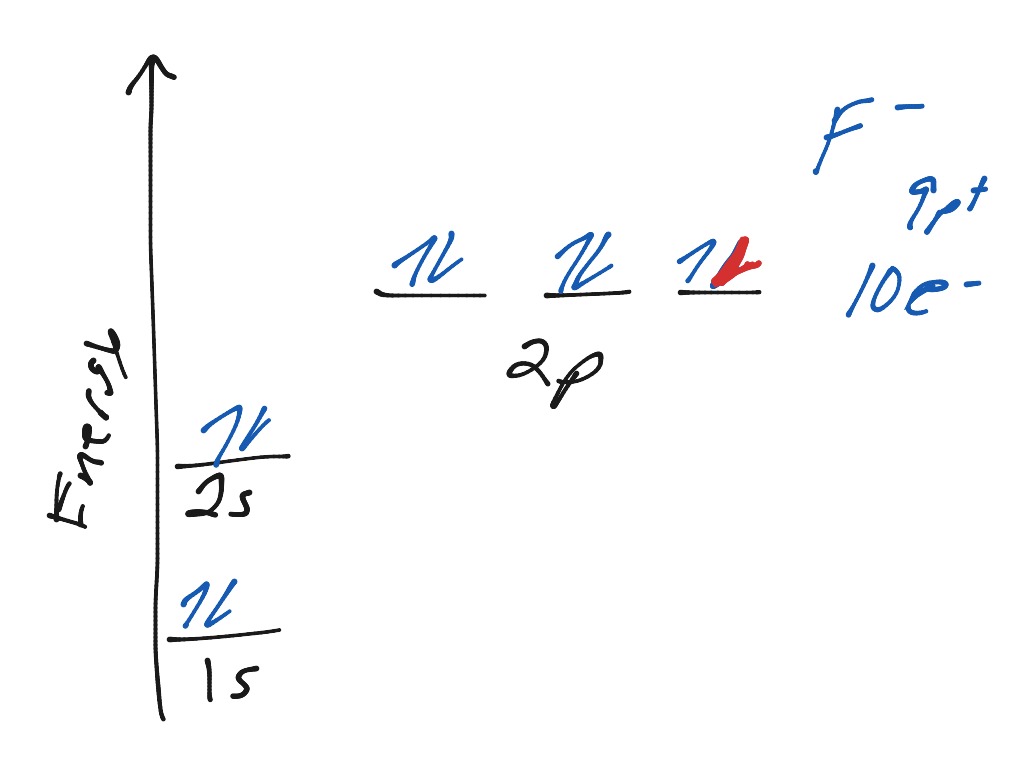











0 Response to "43 orbital diagram of as"
Post a Comment