43 write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic):
An enthalpy diagram is a method used to keep track of the way energy moves during a reaction over a period of time. Learn how to draw and label enthalpy diagrams, the definition of an enthalpy ... (a) Write the balanced equation for the combustion of ethanol to CO 2 (g) and H 2 O(g), and, using the data in Appendix G, calculate the enthalpy of combustion of 1 mole of ethanol. (b) The density of ethanol is 0.7893 g/mL.
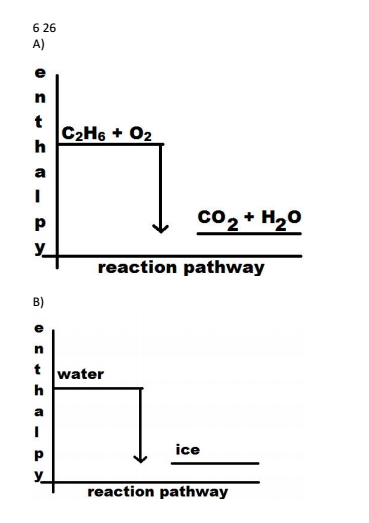
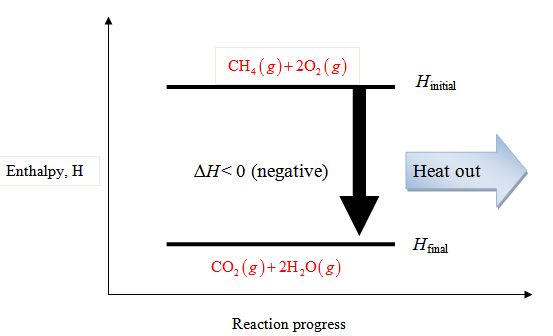
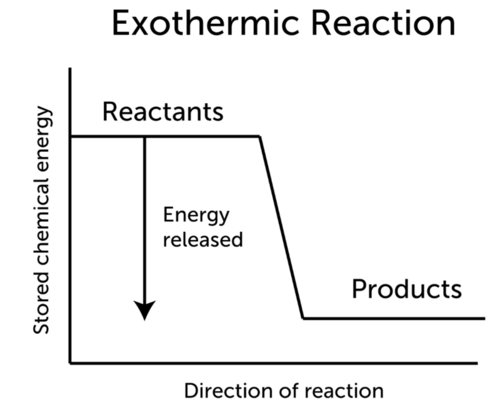
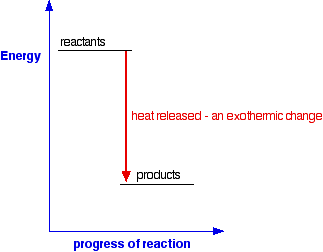
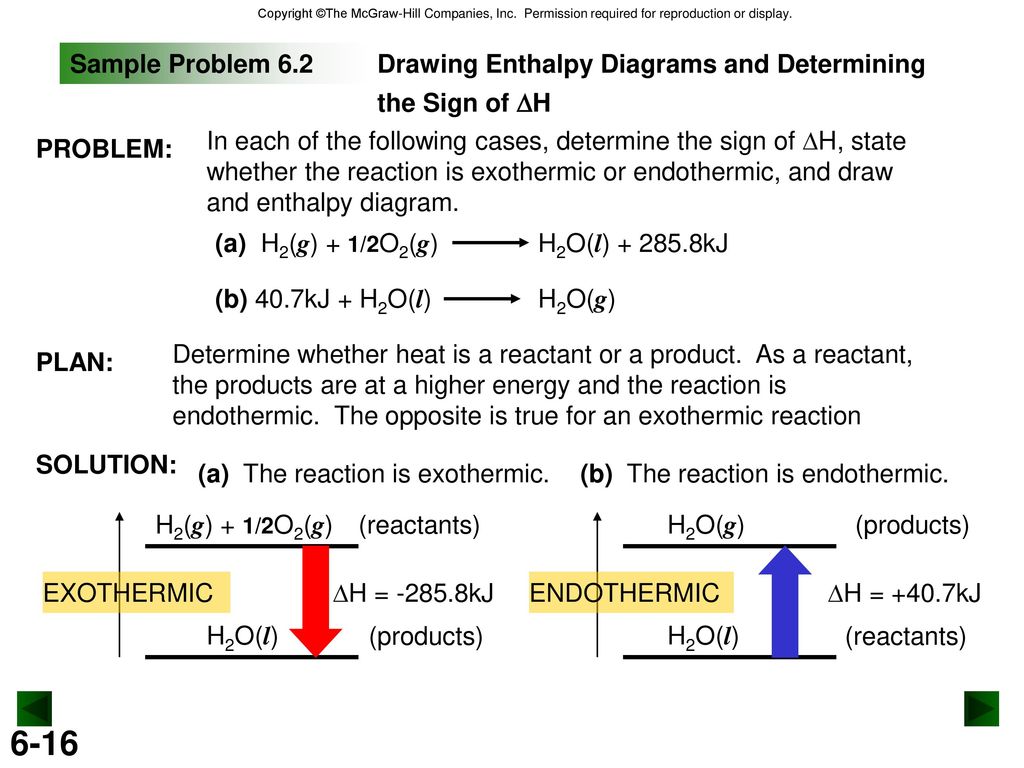
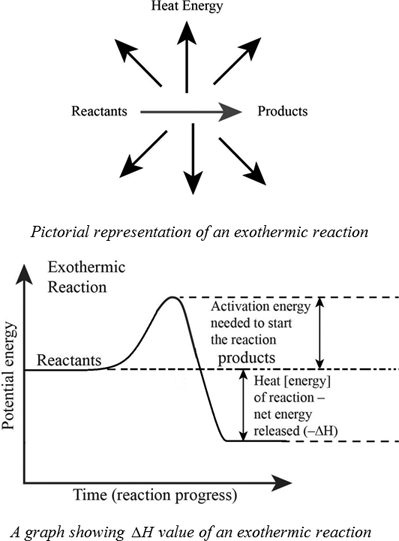
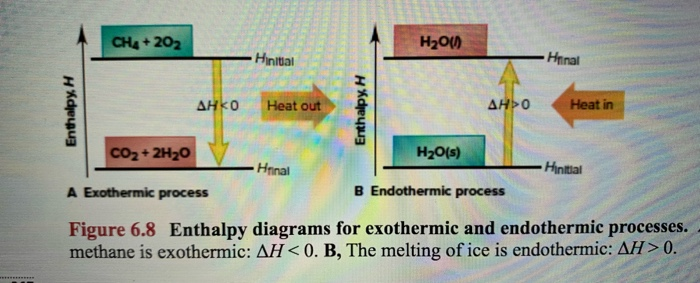
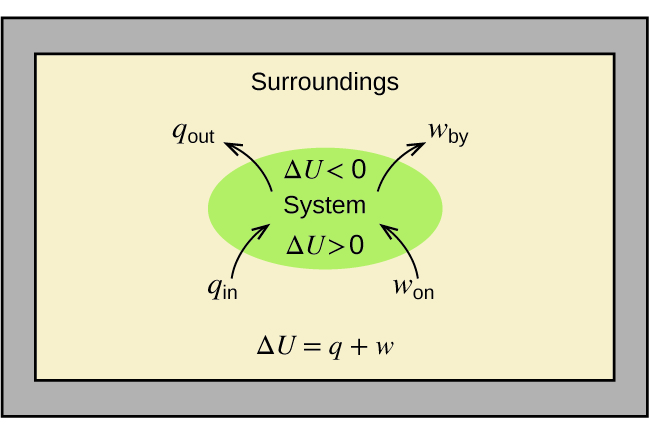
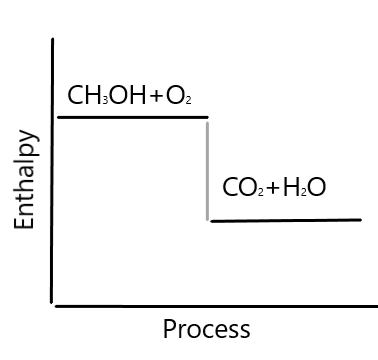
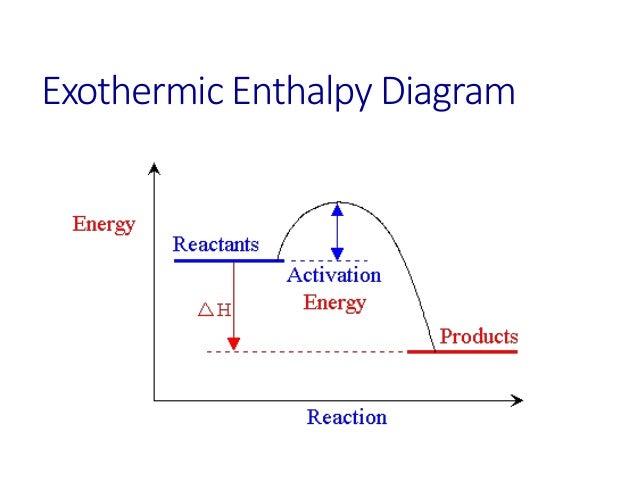
An exothermic reaction is a reaction in which energy is released in the form of light or heat. Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. In an exothermic reaction, change in enthalpy ( ΔH) will be negative.
Write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic):
Solution for Draw a potential energy diagram for a system in which the forward reaction has Eact = +42 kcal/mol and the reverse reaction has Eact = +28… Get the detailed answer: How can one tell from a reaction diagram whether a reaction is exothermic or endothermic? Get the detailed answer: How can one tell from a reaction diagram whether a reaction is exothermic or endothermic? 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → ... Write a balanced equation and draw an enthalpy diagram for(select if exothermic or endothermic):. [ Select ] ["endothermic", "exothermic"] combustion of one ...
Write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic):. UNIT 1 CORRESPONDENCE STUDY PROGRAM PAGE 19 INTRODUCTION TO THERMOCHEMISTRY LESSON 1 Thermochemistry is the study of the change in thermal energy (energy due to the motion of particles). It takes place during physical and chemical changes. Energy is defined as the ability to do work. Work is defined as a force exerted over a distance. There are two types of energy, kinetic Solution for Draw an enthalpy diagram for the following reaction and state whether the reaction is endothermic or exothermic. NaOH + HCl → NaCl + H2O +58 kJ and draw an enthalpic diagram to select if exothermic or endothermic. This because carbon and hydrogen do not react to Make Benzene. Heat flows from the system for fractions (or vice versa); There is a general convention that chemists use in describing and .. write the balanced chemical equation for reaction and identify the appropriate amounts on the board. Discuss demonstration, write the chemical reaction on the board. (Write Equation Here) Lead a discussion on some common exothermic and endothermic reactions. Elicit examples from students and provide additional examples. Examples include: Mixture of cement and water (exothermic) Melting and freezing of ice (endothermic and exothermic)
This investigation introduces the concepts of enthalpy (heat) of ΔH in the context of exothermic and endothermic reactions. To give students a deeper grounding in the basics and reinforce basic concepts covered previously, you may wish to review the mechanics of chemical changes, how to write balanced chemical equations, and the law of ... Write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic): combustion of one mole of methane. vaporization of liquid alcohol. freezing of liquid water. formation of 1 mole of potassium chloride from its elements (heat is released) Learn this topic by watching Energy Diagram Concept Videos. Write. Spell. Test. PLAY. Match. Gravity. Created by. sophiafreiburger. Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. ... Always to the left of the arrow in a chemical equation. In exothermic reactions, there is more energy in the reactants than in the products. Exothermic processes release energy upon completion, and are signified by a negative change in enthalpy. Terms. exothermicOf a chemical reaction that releases ...
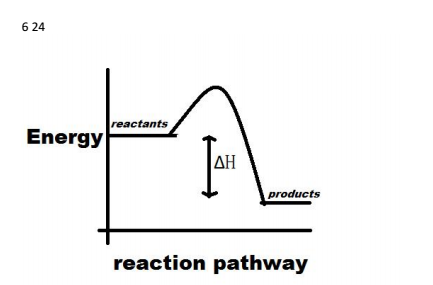
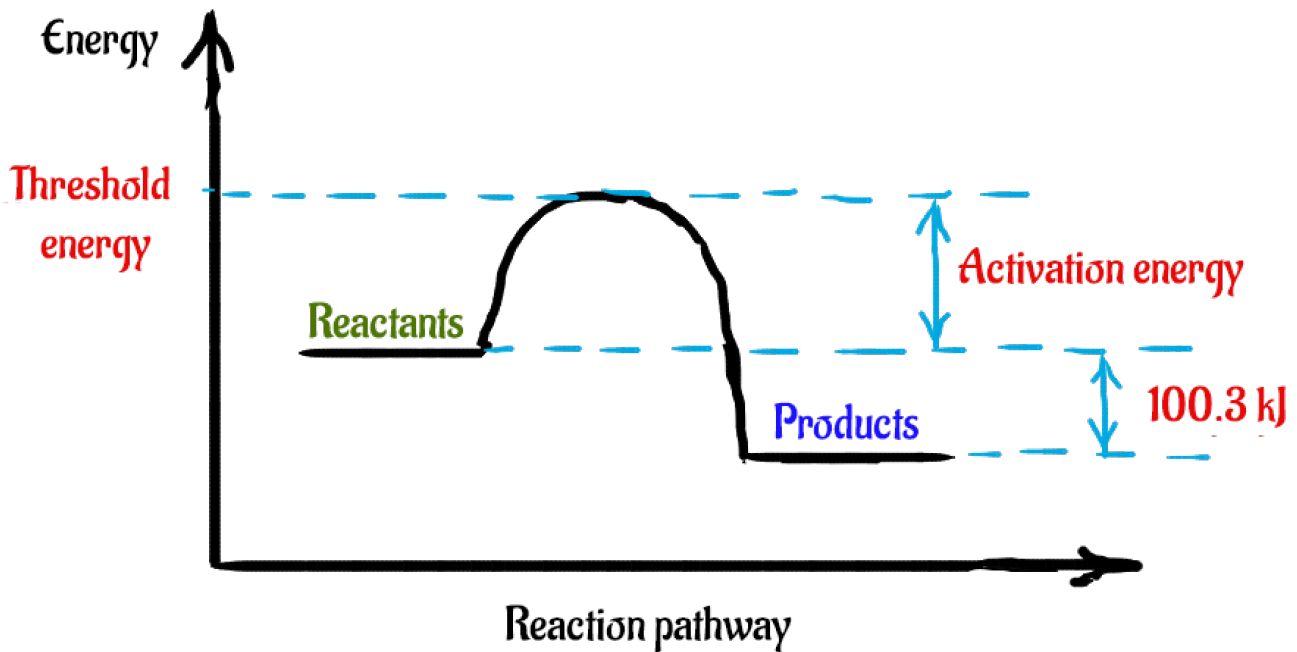
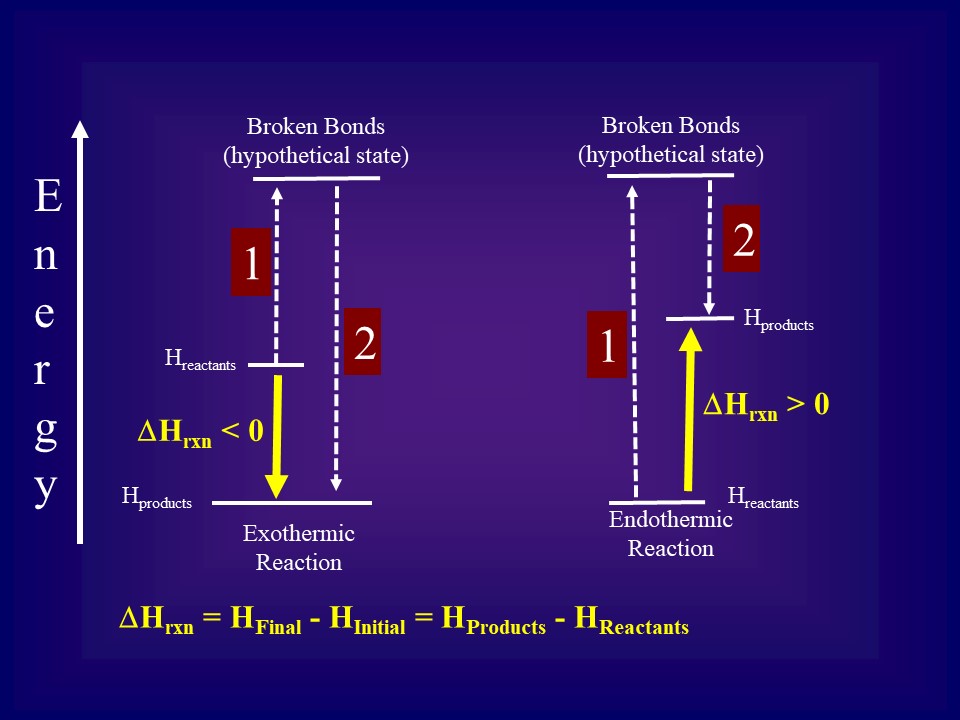
9. Draw an enthalpy diagram for a general exothermic reaction; label axis, reactants, products, and ∆H with its sign. 10. Draw an enthalpy diagram for a general endothermic reaction; label axis, reactants, products, and ∆H with its sign. 11. Write a balanced equation and draw an approximate enthalpy diagram for each of Heat of reaction is given the symbol AH and is usually measured in kilojoules (kJ). ΔH = Total energy content of products - total energy content of reactants = H products - H reactants In an exothermic reaction, the reactants lose heat energy to form products. Thus, the products formed have less energy than the reactants, H products < H reactants. Write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic):. [ Select ] ["endothermic", "exothermic"] combustion of ... Q. Write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic):combustion of one mole of methanevaporization of l... Solved • Apr 22, 2020 Energy Diagrams
Thermochemistry 1. 6.1 Basic principles 6.2 measurement of heat flow- Calorimetry (Laboratory Measurement of Heats of Reaction) 6.3 Energy and Changes of State 6.4 The first law of thermodynamics 6.5 Enthalpy change for chemical reaction 6.6 Hess's Law 6.7 Standard enthalpy of formation 6.8 Product or reactant-favored reaction and thermochemistry
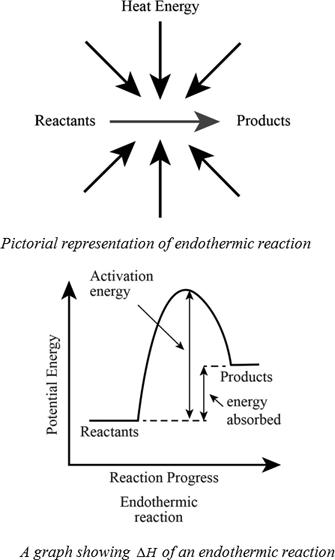
An exothermic process releases heat, causing the temperature of the immediate surroundings to rise. An endothermic process absorbs heat and cools the surroundings.". Based on the above definition, let's pick a few examples from our daily lives and categorize them as endothermic or exothermic.
3:04 calculate the molar enthalpy change (ΔH) from the heat energy change, Q; 3:05 (Triple only) draw and explain energy level diagrams to represent exothermic and endothermic reactions; 3:06 (Triple only) know that bond-breaking is an endothermic process and that bond-making is an exothermic process
If heat is given off, it is called an exothermic reaction. If heat is absorbed, it is called endothermic reaction. In this chapter we will learn how to calculate the enthalpy change (heat change) when methane (a gas) is formed using carbon graphite and hydrogen. Hess's law. Hess's law is used to determine the enthalpy change.
Write balanced chemical equations including heat energy. Identify processes as either exothermic or endothermic based on evidence, such as temperature changes in a chemical reaction. Associate an endothermic and exothermic chemical reaction with their related energy diagram. Understand how a chemical reaction is related to an energy diagram.
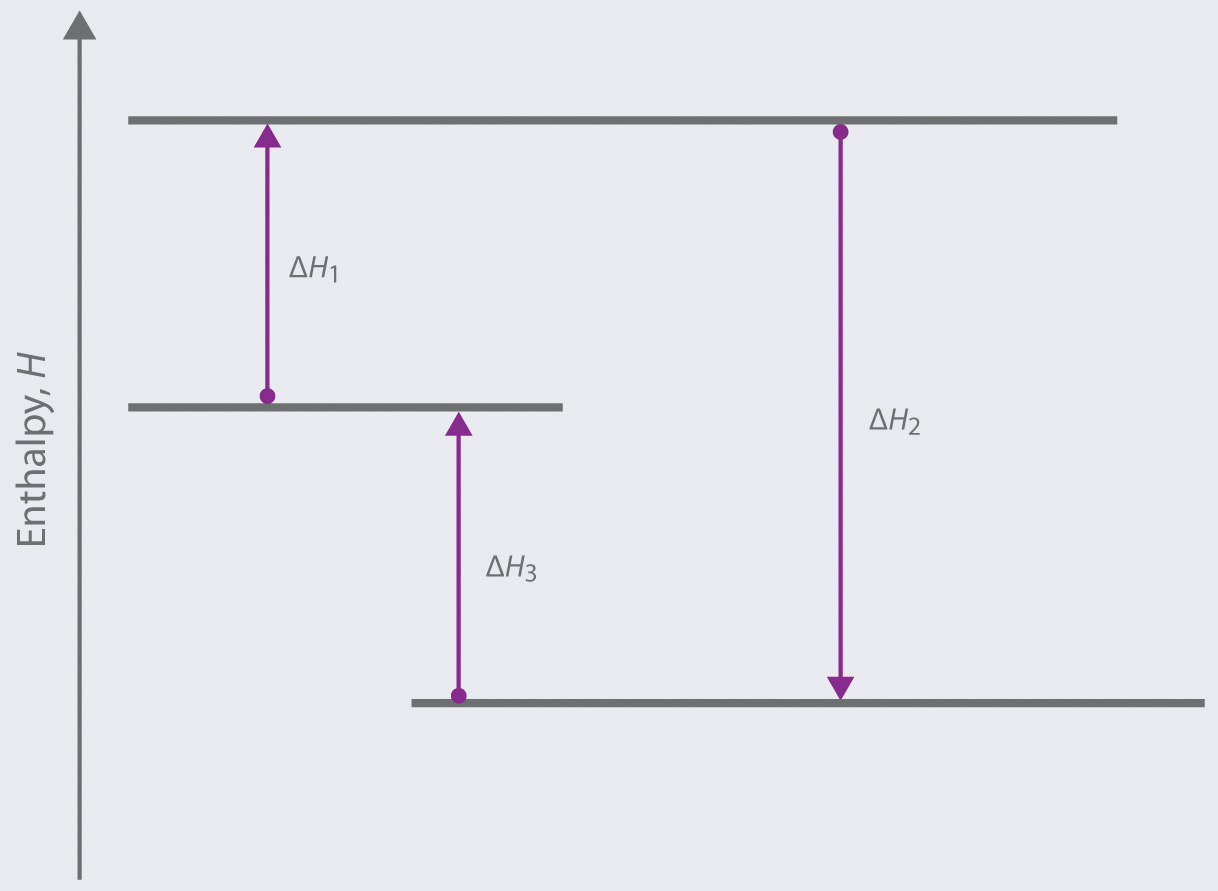
Stoichiometry of Thermochemical Equations • A thermochemical equation is a balanced equation that includes DHrxn. • The sign of DH indicates whether the reaction is exothermic or endothermic. • The magnitude of DH is proportional to the amount of substance. • The value of DH can be used in a calculation in the same way as a mole ratio.
Use the formula ∆H = m x s x ∆T to solve. Once you have m, the mass of your reactants, s, the specific heat of your product, and ∆T, the temperature change from your reaction, you are prepared to find the enthalpy of reaction. Simply plug your values into the formula ∆H = m x s x ∆T and multiply to solve. How do you draw a reaction energy diagram?
Write the balanced thermochemical equation for the combustion of methane gas, CH 4(g). b. Draw a potential energy diagram that would reasonably represent this combustion reaction. Indicate the Δ H comb and a molecular structure that could represent an activated complex in this potential energy diagram. What Is Required?
Write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic): [ Select ] ["endothermic", "exothermic"] combustion of one mole of methane [ Select ] ["endothermic", "exothermic"] vaporization of liquid alcohol
Endothermic: E reactants < E products. 86% (56 ratings) ... Q. Write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic):combustion of one mole of methanevaporization of ...
Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react.
write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic):. Answer. +20. Watch. 1. answer. 0. watching. 88. views.
This problem has been solved! Write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic): [ Select ] ["endothermic", "exothermic"] combustion of one mole of methane. [ Select ] ["endothermic", "exothermic"] vaporization of liquid alcohol.
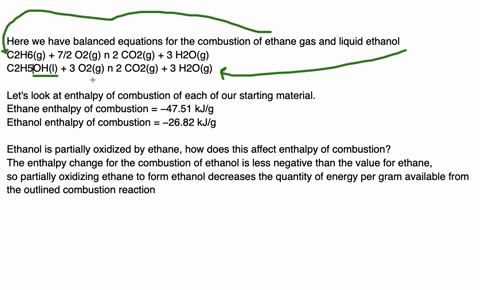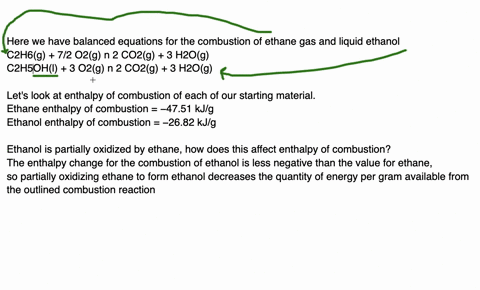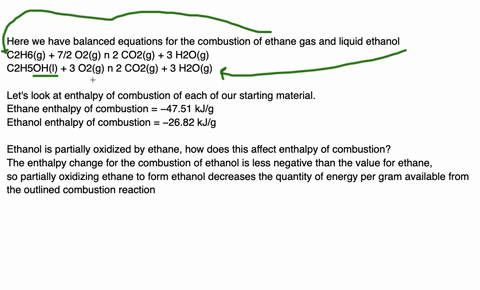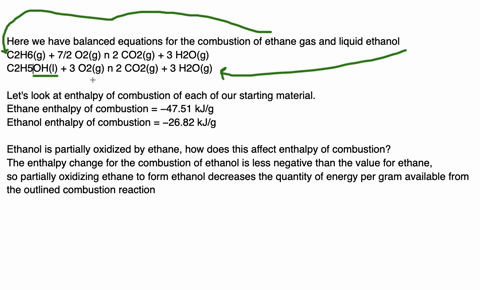
Since enthalpy is measured in kJ/mol, the coefficients in the equation represent the amount in moles of each reacting substance. e.g. 2H2 (g) + O2 (g) ==> 2H2O (l) delta H = -572 kJ/mol Can be read as: when 2 moles of hydrogen react with 1 mole of oxygen, 2 moles of water form and 572 kJ of energy is produced.
Write a balanced equation and draw an enthalpy diagram for(select if exothermic or endothermic):. [ Select ] ["endothermic", "exothermic"] combustion of one ...
Get the detailed answer: How can one tell from a reaction diagram whether a reaction is exothermic or endothermic? Get the detailed answer: How can one tell from a reaction diagram whether a reaction is exothermic or endothermic? 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → ...
Solution for Draw a potential energy diagram for a system in which the forward reaction has Eact = +42 kcal/mol and the reverse reaction has Eact = +28…











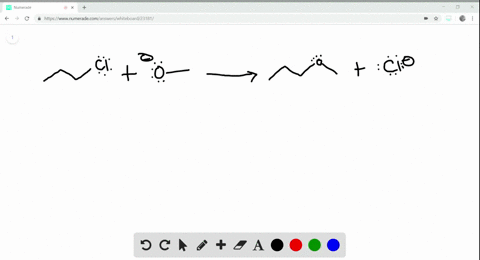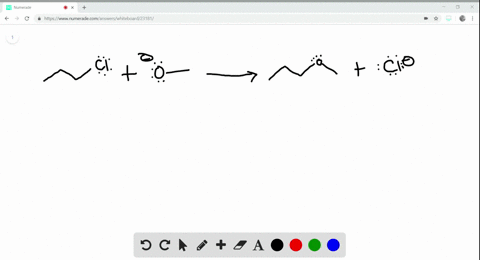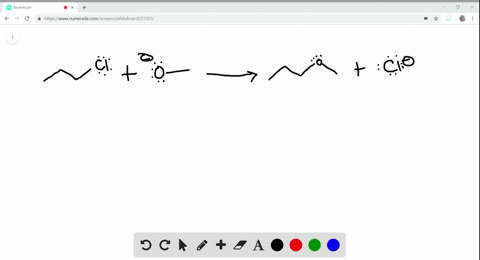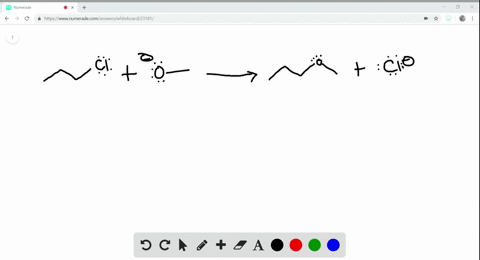


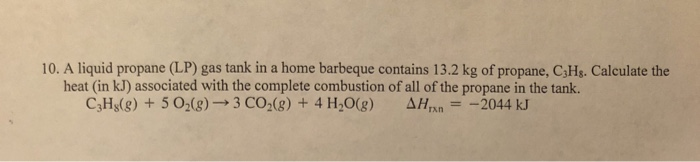
:max_bytes(150000):strip_icc()/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)




0 Response to "43 write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic):"
Post a Comment