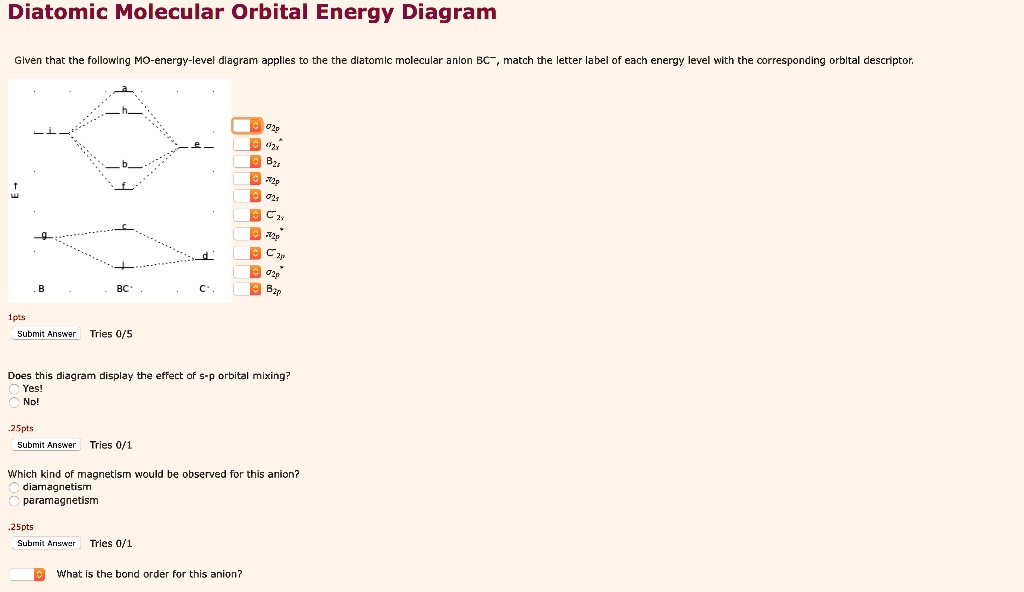
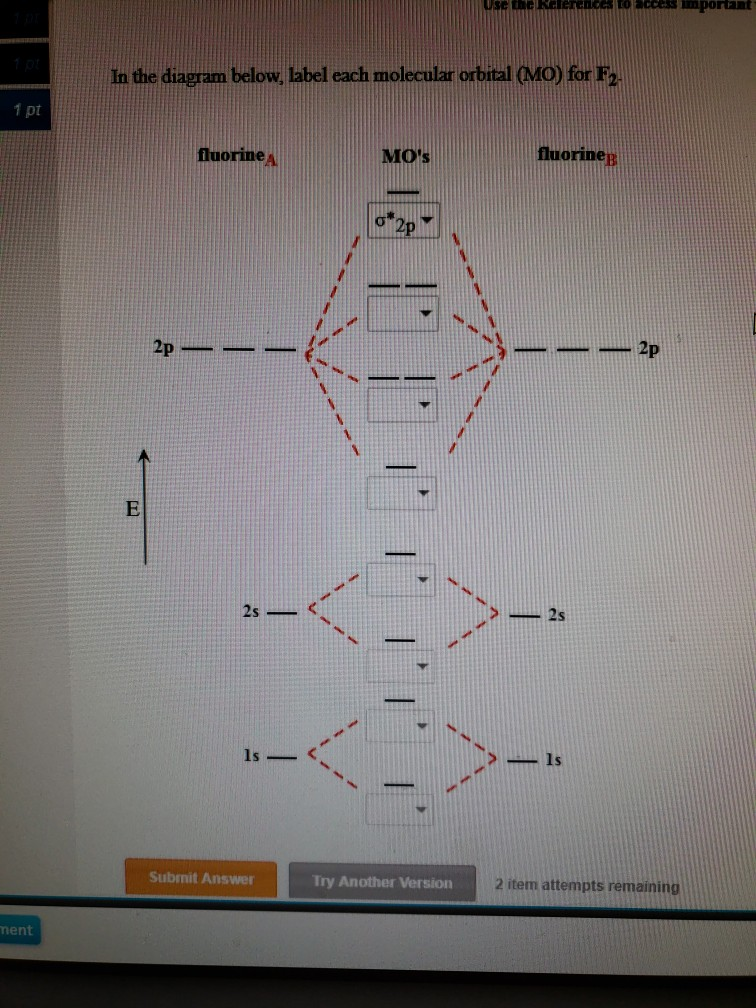
44 label the various areas in the molecular orbital diagram by moving the labels onto the diagram.
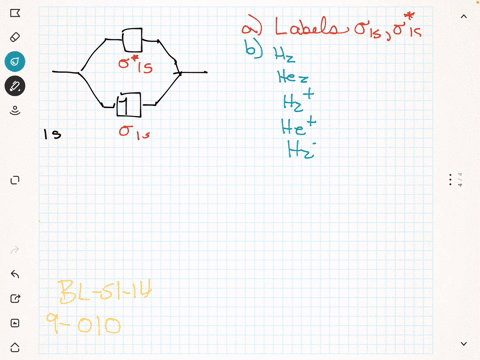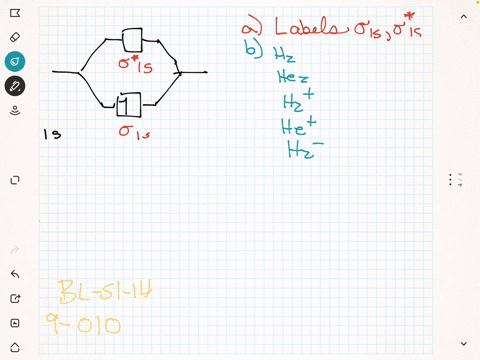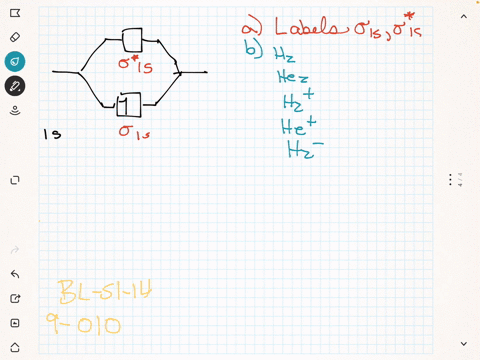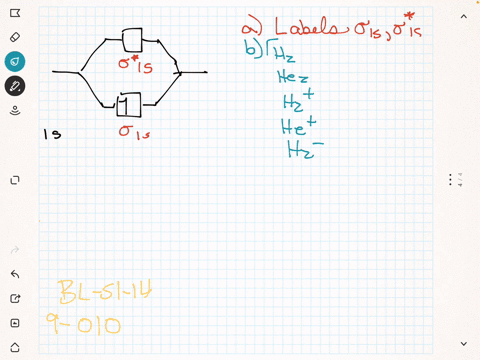
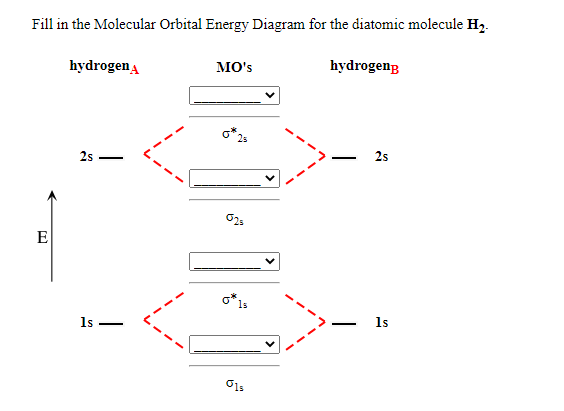
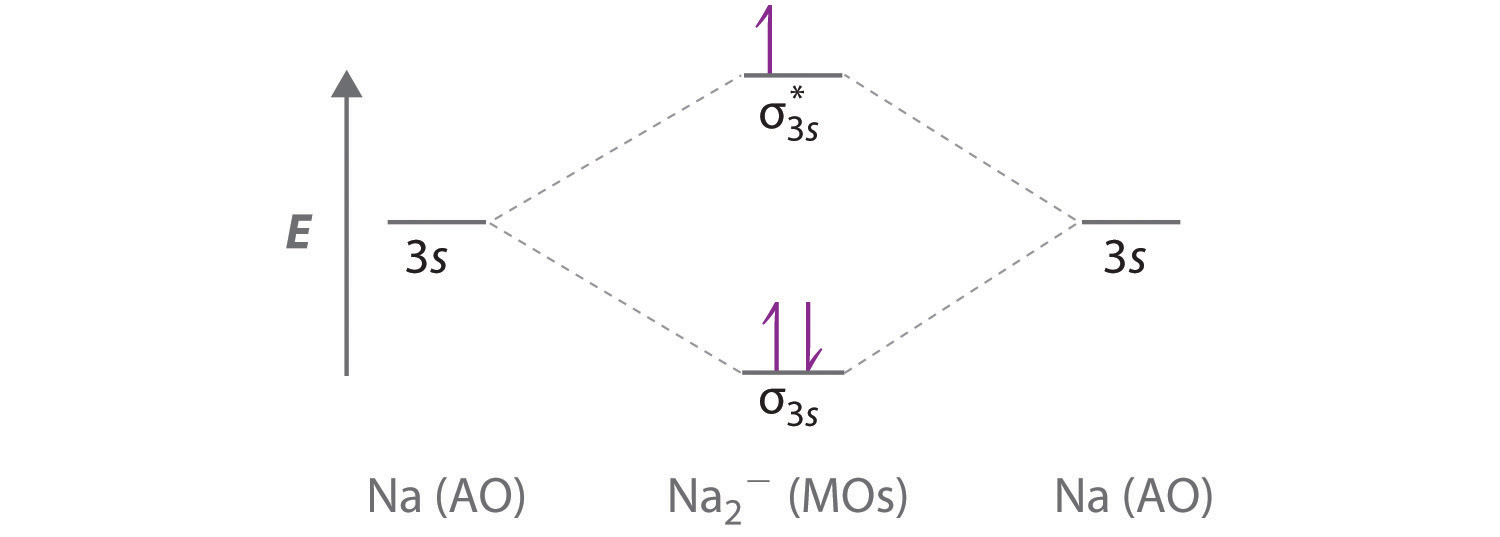
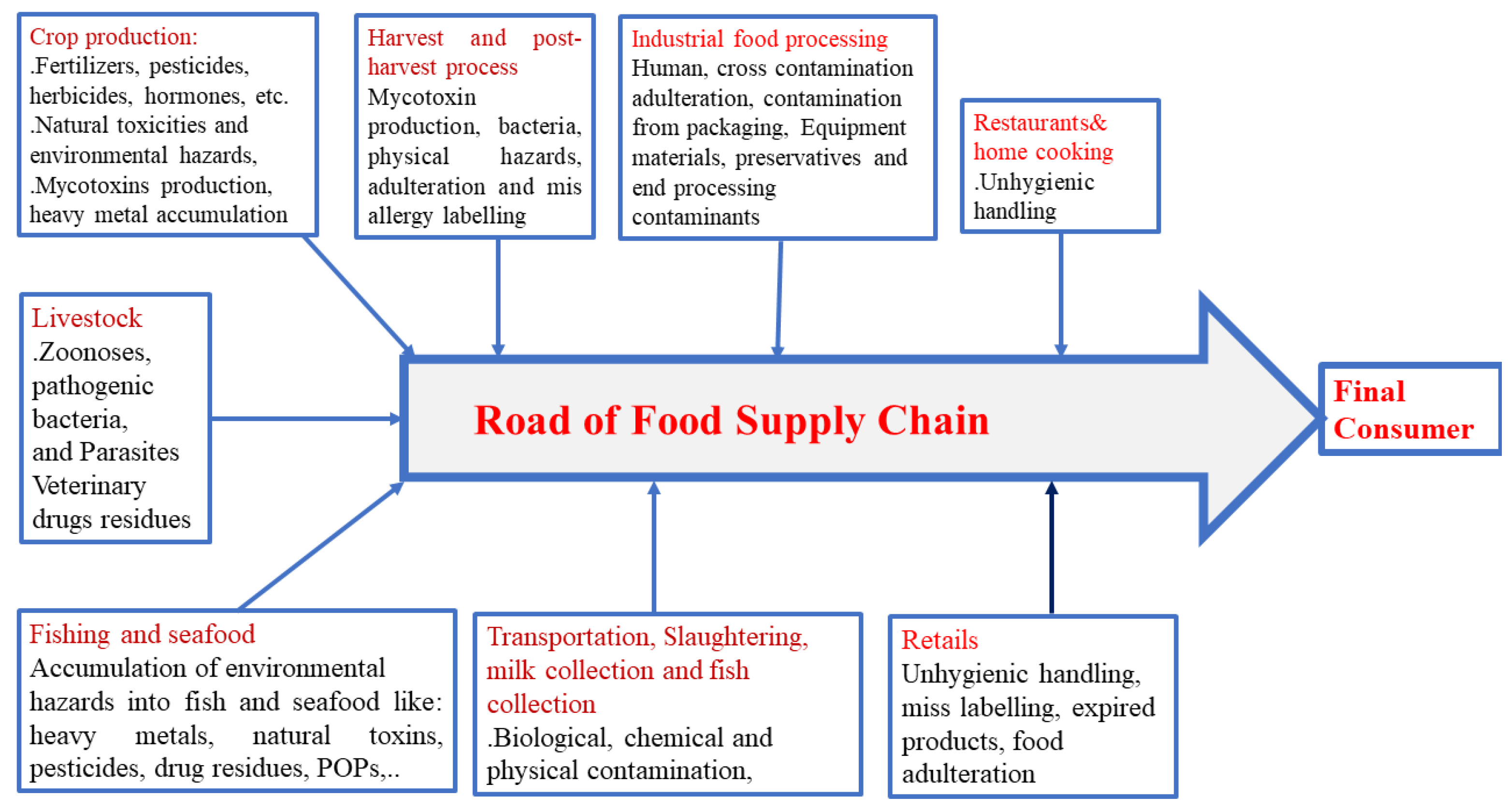
Label the various areas in the molecular orbital diagram by moving the labels onto the diagram.. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion h 2. Each horizontal line represents one orbital that can hold two electrons. On the periodic table, hydrogen and helium are the only two elements in the first row, or period, which reflects that they only have electrons in their first shell. Hydrogen and helium are the only two elements that have electrons exclusively in the orbital in their neutral, non-charged, state. The second electron shell, 2n, contains another ...
To answer the question, we should display the molecular orbital with the highest energy, the so-called Highest Occupied Molecular Orbital or HOMO for short. Electron deficient species interact with the electrons in this orbital the most and hence of all the molecular orbitals in the molecule, the HOMO is most indicative of the species chemistry.

Label the various areas in the molecular orbital diagram by moving the labels onto the diagram.
An orbital is also an area of space in which an electron will be found 90% of the time. Orbitals have a variety of shapes. Each orbital has a characteristic energy state and a characteristic shape. The s orbital is spherical. Since each orbital can hold a maximum of two electrons, atomic numbers above 2 must fill the other orbitals. Academia.edu is a platform for academics to share research papers. A molecular formula is the simplest way to represent a compound by counting up all of the different types of atoms and listing them in order. For example, the sugar glucose, contains 6 carbons, 12 hydrogens, and 6 oxygens. The molecular formula would then be written as C 6 H 12 O 6. By convention, carbon is listed first, hydrogen second ...
Label the various areas in the molecular orbital diagram by moving the labels onto the diagram.. The mitosis and meiosis Venn diagram graphic organizer is a great way for students to compare and contrast the characteristics of these two cellular processes.Includes two versions:Version 1. Students cut and paste labels onto the correct part of the Venn diagram.Version 2. Students write the correc MolView is an intuitive, Open-Source web-application to make science and education more awesome! Molecular Orbital Diagram Maker. These quizzes enable you to build your own molecular orbital diagram from components. A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. Above the drawing area, there is a text field which displays the current structure in various encodings useful for cutting and pasting. It can also be used to input structure information into the sketcher from structure encodings on the clipboard, or even by typing in structure codes by advanced users.
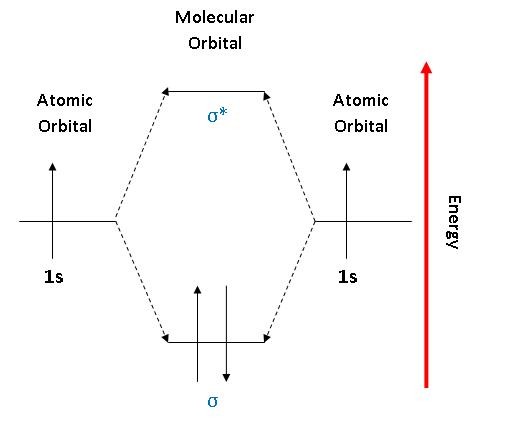
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in Before electrically testing the switch, check the loc- Purpose ation of the switch and bolt, to make sure they are meeting in the sense zone on the switch. Both the switch and the bolt are adjustable and the air gap between them should be 1/8" to 1/4" ( to mm). 2. Wheel Horse Ignition Switch Wiring Diagram. Academia.edu is a platform for academics to share research papers. Wow, the Quad Xeon is the Pentium D. diagram without labels. heart diagram with labels. statesheep heart labels; statesheep heart labels. Vulpinemac. Apr 28, 09:47 AM. Almost all of that is due to the iPad. Heart+diagram+to+label. Now that we have converted the heart into a square with 4 different boxes or chambers, the heart can be divided ...
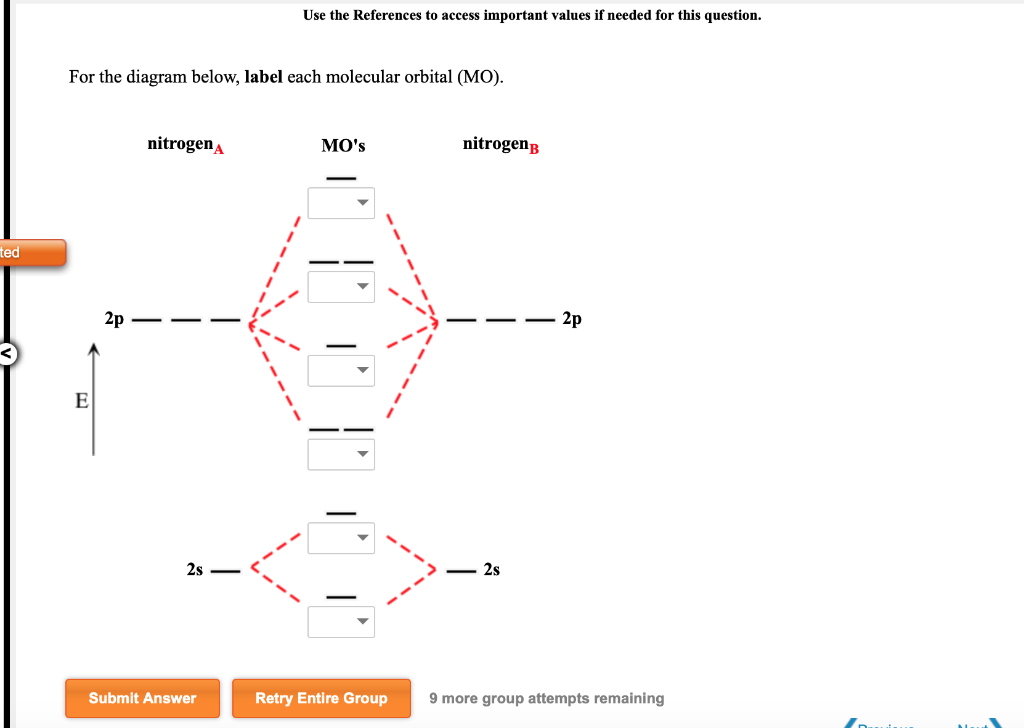
The molecular orbitals are obtained by allowing the orbitals of the M and L6 fragments to interact (Figure 2.2). We note features that were established in 2.1.1: the formation of six bonding MO (1a1g , 1t1u , and 1eg ), by bonding interactions involving the fragment orbitals whose symmetries are a1g , t1u , and eg , and the formation of six ... Question: Label the various areas in the molecular orbital diagram by moving the labels onto the diagram. v 2nd attempt A empty molecular orbitals B occupied molecular orbitals C overlap D valence band Econduction band The first known pair of these molecules were two different types of tartaric acid whose behavior was explained in 1844 by Louis Pasteur. 30 years later, van't Hoff and le Bel deduced that the atoms of fluorochlorobromomethane must be arranged in a tetrahedron to explain the different "handedness" of its two forms, which also fall in this category. The following diagram illustrates the kind of evidence considered, although some of the reagents shown here are different from those used by the original scientists. Hot hydriodic acid (HI) was often used to reductively remove oxygen functional groups from a molecule, and in the case of glucose this treatment gave hexane (in low yield).
moving left, d-obitals fill up - held loosely to nucleus Malleable and very conductive comes in varying positive oxidation states: because electrons from s and d orbitals easily lost Because of many positive oxidation states, forms lots a lot of ionic compounds Dissolved ions can form COMPLEX IONS with water (Hydration Complexes) or with non-metals
Drag labels onto the provided image. Sometimes a label can be used more than once, or it may not be used at all for the correct answer. When you're satisfied with your answer, select Submit.. If you can't drag one or more labels to an incorrect target, try to position the label on another target.. To clear all your labels you've placed, select Reset (next to Help).
In atomic theory and quantum mechanics, an atomic orbital is a mathematical function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus.The term atomic orbital may also refer to the physical region or space where the electron can be ...
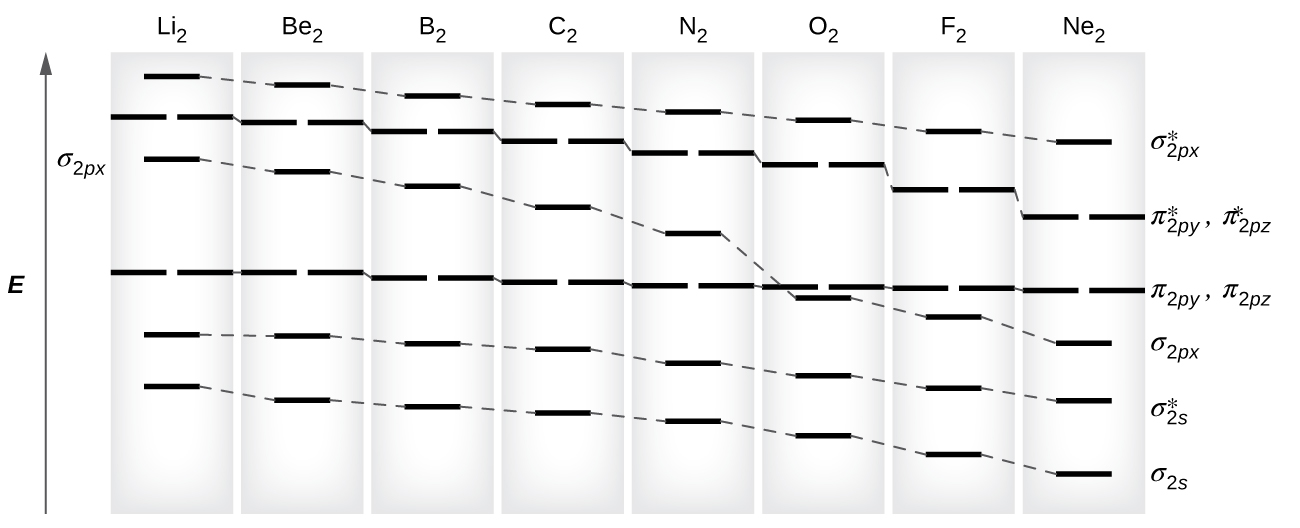
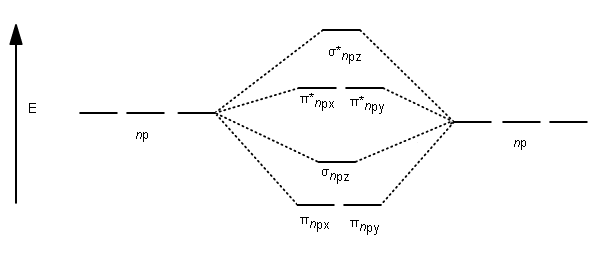
A reasonably-accessible discussion of molecular orbitals, including a sketch of the energy-level diagram for O 2, can be found in reference 2. Another useful source is reference 12, which contains energy-level diagrams for some interesting molecules (water, hydrogen fluoride, ammonia, methane, ethane, ethene, ethyne).
The motion of objects is determined by the relative size and the direction of the forces that act upon it. Free-body diagrams showing these forces, their direction, and their relative magnitude are often used to depict such information. In this Lesson, The Physics Classroom discusses the details of constructing free-body diagrams. Several examples are discussed.
Like the mixing of atomic orbitals on different atoms to form molecular orbitals, the mixing of atomic orbitals on the same atom forms new hybrid orbitals. When we mix the 2s and one of the 2p wave functions on beryllium, we obtain two new hybrids, called sp orbitals, made up of 50% s and 50% p character.
In a pulley system two masses are strung over a pulley. Free Body Diagram of Cable-Pulley System C The system is held in equilibrium at angle Θ by the tension, T. 30º *Click to see solutions A cable connects pulley A to the ceiling at point C. A Θ T Ignore the weight of the pulley and the cable.
Clean up your area. Questions. 1. Recall the different states of matter (solid, liquid, gas). How do the water molecules differ in the liquid and gas states? Draw and explain. 2. Plot your temperature and time data on the graph below. 3. Label the phase changes on your heating curve above. 4. What happens to the molecules as they begin to boil? 5.
A molecular formula is the simplest way to represent a compound by counting up all of the different types of atoms and listing them in order. For example, the sugar glucose, contains 6 carbons, 12 hydrogens, and 6 oxygens. The molecular formula would then be written as C 6 H 12 O 6. By convention, carbon is listed first, hydrogen second ...
Academia.edu is a platform for academics to share research papers.
An orbital is also an area of space in which an electron will be found 90% of the time. Orbitals have a variety of shapes. Each orbital has a characteristic energy state and a characteristic shape. The s orbital is spherical. Since each orbital can hold a maximum of two electrons, atomic numbers above 2 must fill the other orbitals.










![Co(NH3)6]3+ion 4. Construct the MO diagram. Label all atomic ...](https://img.homeworklib.com/questions/54d51fd0-7560-11ea-b11c-e96f4202d848.png?x-oss-process=image/resize,w_560)
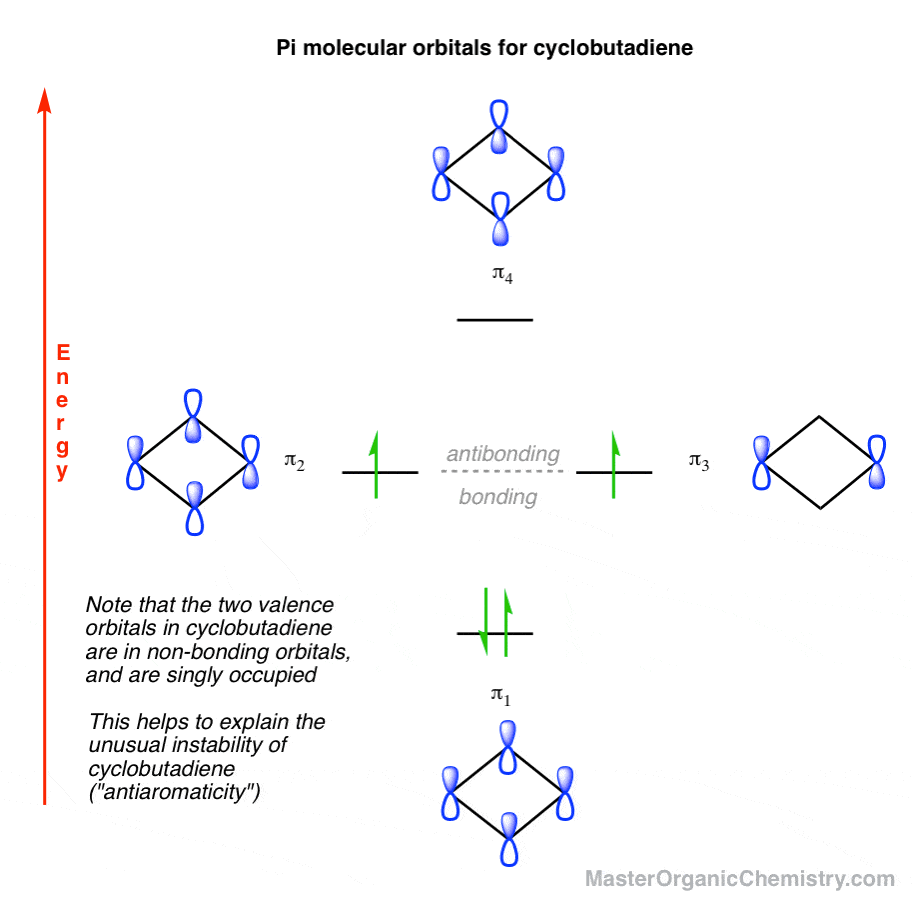

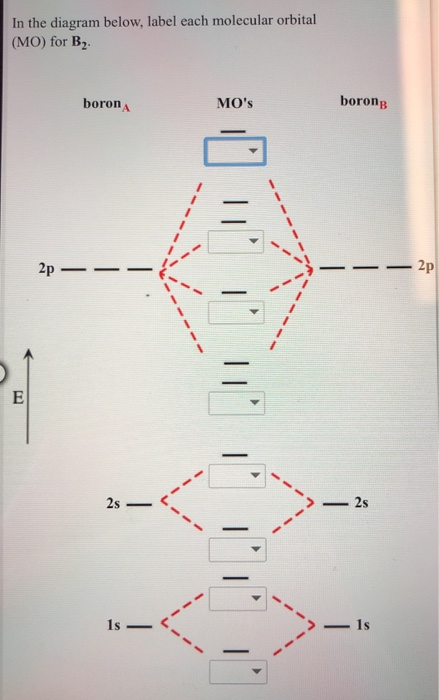




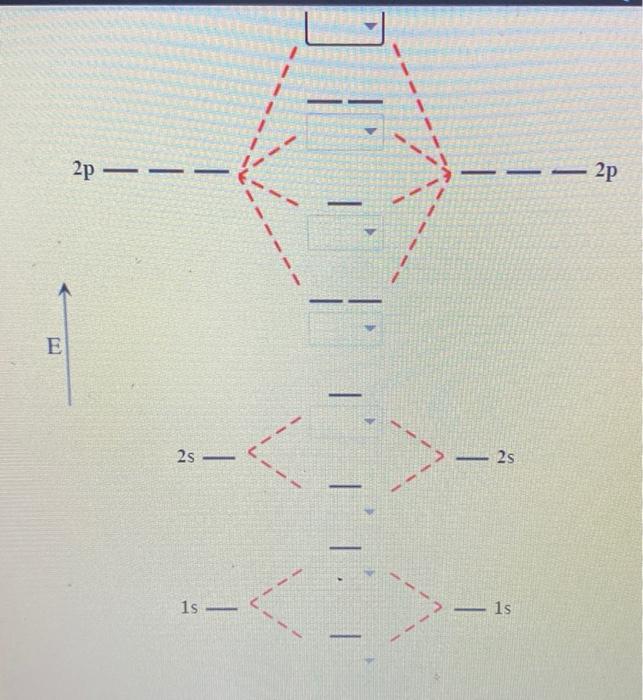

0 Response to "44 label the various areas in the molecular orbital diagram by moving the labels onto the diagram."
Post a Comment