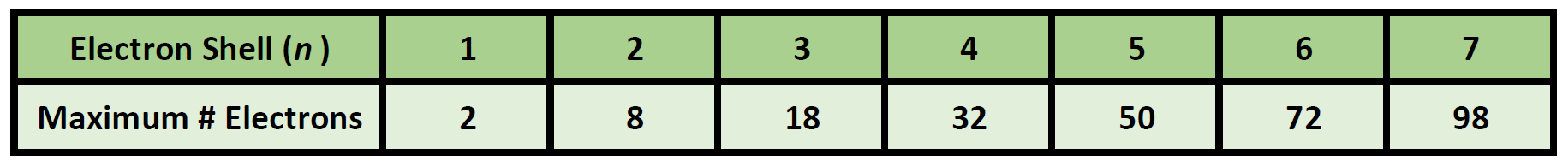
44 what is the maximum number of electrons that can occupy a box in an orbital filling diagram
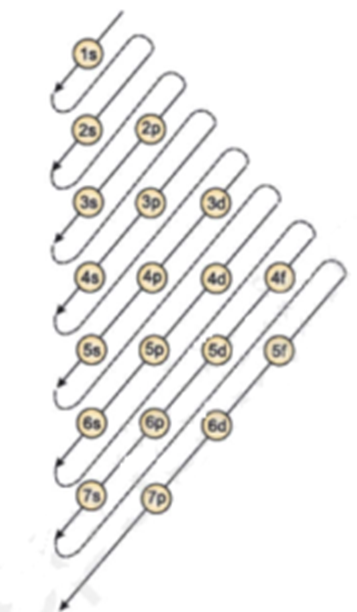
What is the maximum number of electrons that can occupy ... There are five 3d orbitals, each of which can hold up to 2 electrons, for 10 total electrons. An orbital is described by the principle quantum number, n, the angular momentum quantum number, l, and the magnetic quantum number, m_l. An electron is described by each of these quantum numbers, with the addition of the electron spin quantum number, m_s. Chemistry 2.12: Electron Orbitals Flashcards | Quizlet Chemistry 2.12: Electron Orbitals. What is the maximum number of electrons that can occupy a box in an orbital filling diagram at any energy level? What is the maximum number electrons that can occupy any d orbital? Use an aufbau diagram.
What is the maximum number of electrons that can occupy a ... What is the maximum number of electrons that can occupy a box in an orbital filling diagram at any energy level? A. 2 B. 6 C. 8 D. 14

What is the maximum number of electrons that can occupy a box in an orbital filling diagram
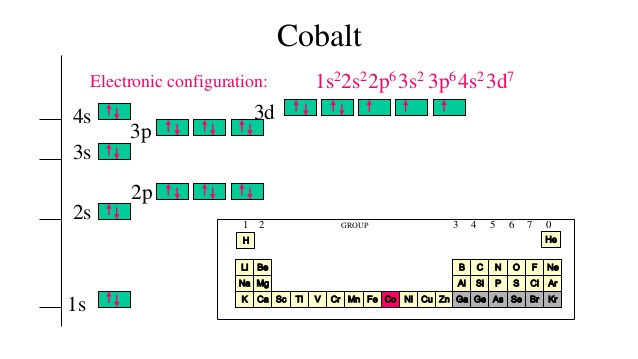
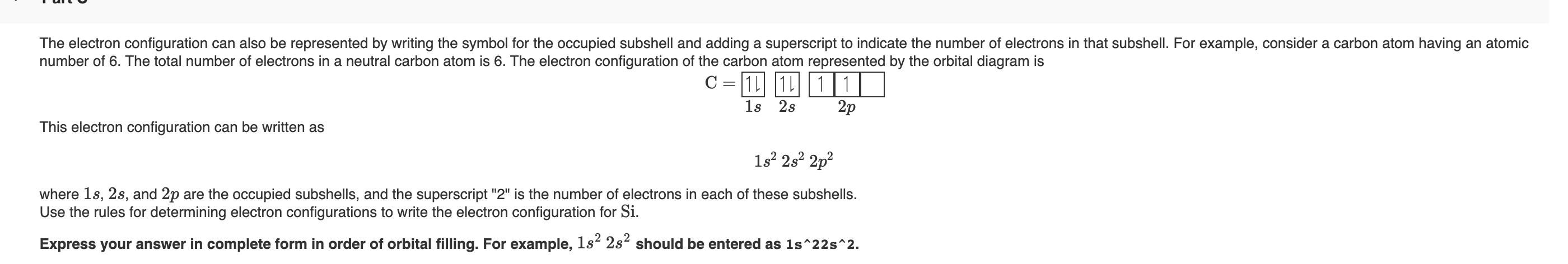
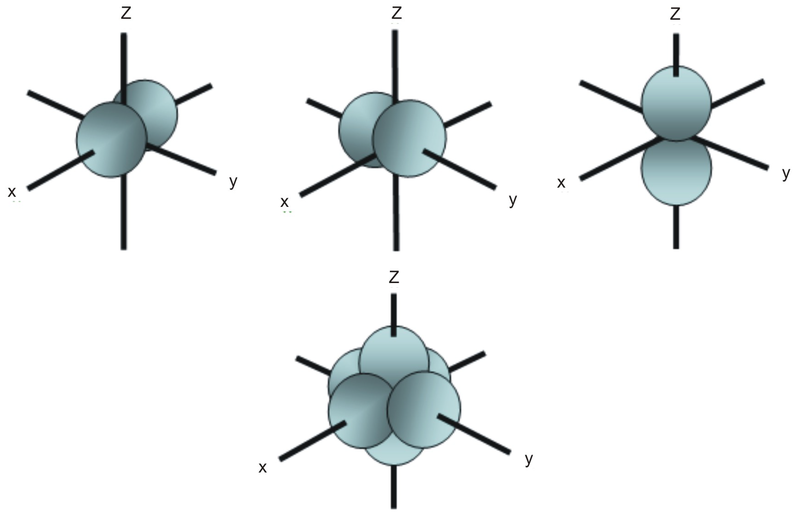
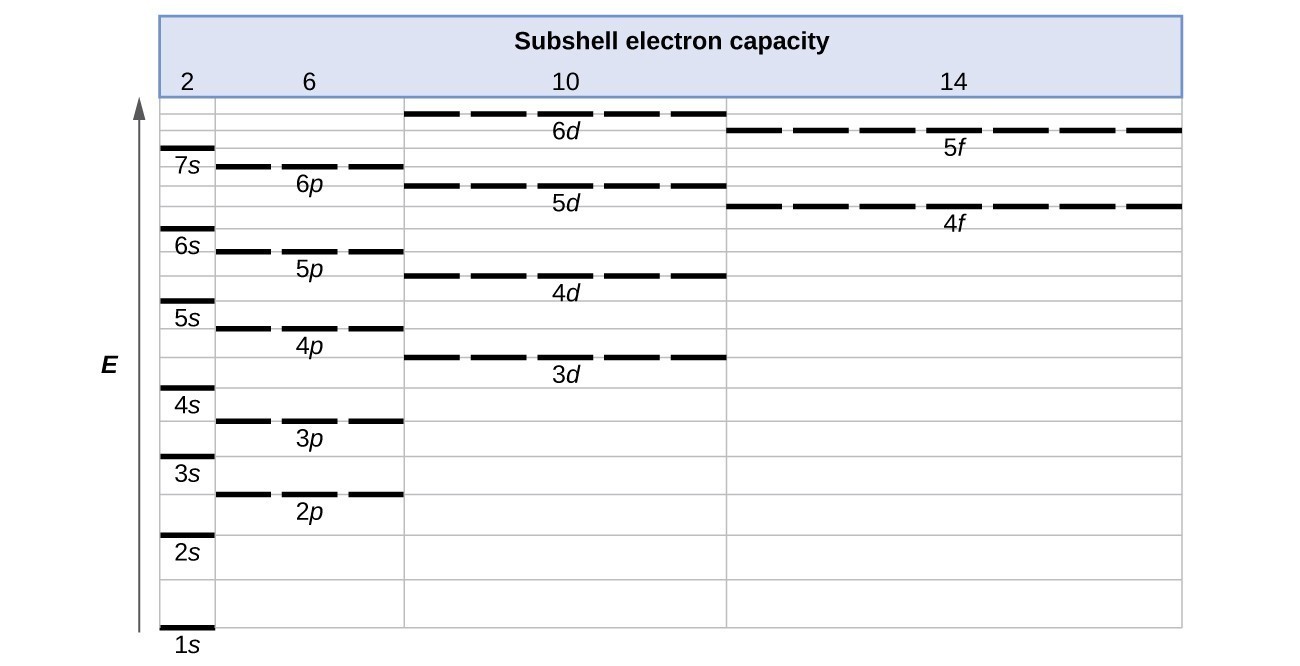
What is the maximum number of electrons that can occupy a ... The major difference between a 1s orbital and a 2s orbital is that; The electrons that occupy the outermost filled shell are called; When filling degenerate orbitals, electrons fill them singly first, with parallel spins is known as; You can click the format cells dialog box launcher to open the format cells dialog box. What Is Used To Represent An Orbital In An Orbital ... Electron Configuration Standard Notation The number and letter describe the energy level and orbital, and the number above the orbital shows how many electrons are in that orbital. Using standard notation, the electron configuration of fluorine is 1s22s22p5. What do the arrows in orbital notation represent? An orbital diagram, or orbital box ... Argon Orbital diagram, Electron configuration, and Valence ... S orbital contain 1 box that can hold a maximum of 2 electrons. P orbital contains 3 boxes that can hold a maximum of 6 electrons. D orbital contains 5 boxes that can hold a maximum of 10 electrons. F orbital contains 7 boxes that can hold a maximum of 14 electrons. The orbital diagram will also be filled with the same order as described by the ...
What is the maximum number of electrons that can occupy a box in an orbital filling diagram. 39 what is the maximum number of electrons that can occupy ... An orbital can occupy a maximum of two electrons. e.g. Electronic Configuration of Ions What is the maximum number of electrons that can occupy a box in an orbital filling diagram at any energy level? → 6 → 14 → 8 → 2. 2. what is the maximum number of electrons that can occupy a ... Each orbital can hold a maximum of 2 electrons, and only if the electrons have opposite spins (Pauli exclusion principal). Electrons with the same spin must occupy each equal-energy orbital before additional electrons with the opposite spins can occupy the same orbitals (Hund's Rule). Chemistry 2.12 Quiz - Chemistry 2.12 Quiz Electron ... Chemistry 2.12 Quiz: Electron Orbitals ion 1 1 / 1 point What is the maximum number of electrons that can occupy a box in an orbital filling diagram at any energy level? 8 2 6 14 View Feedback ion 2 1 / 1 point Look at an aufbau diagram. What is the maximum number of electrons that can go into ... The maximum number of electrons in any s sublevel is 2, irrespective of what the principal quantum number, i.e., the number before the s in an electron configuration, may be.
2.11 Quiz: Electron Orbitals Flashcards | Quizlet 2.11 Quiz: Electron Orbitals. What is the maximum number of electrons that can occupy a box in an orbital filling diagram at any energy level? What is the maximum number electrons that can occupy any d orbital? Use an aufbau diagram. © What is the maximum number of electrons that can occupy ... Register Now. Lorem ipsum dolor sit amet, consectetur adipiscing elit.Morbi adipiscing gravdio, sit amet suscipit risus ultrices eu.Fusce viverra neque at purus laoreet consequa.Vivamus vulputate posuere nisl quis consequat. Elements that are characterized by the filling of p ... What is the maximum number of electrons that can occupy a box in an orbital filling diagram; A(n) ____ contains instructions for filling areas of a template. Which of the following option buttons gives options for filling cells following a fill operation; What must be done to the refrigerant that is vented off the top when filling a charging ... What Is The Maximum Number Of Electrons That Can Occupy A ... Individual orbitals can hold no more than 2 electrons each, regardless of the type of orbital you've got. The d -subshell is comprised of 5 d - orbitals, which means it can hold a maximum of 10 electrons, 2 from each d - orbital.
q6.docx - According to quantum mechanics, what is the ... According to quantum mechanics, what is the maximum number of electrons that may occupy a 5f orbital Place your answer in the box. Express your answer using only a number without text. 2 Indicate which of the following statements is false a) Zn is paramagnetic b) Ga is paramagnetic c) Na is paramagnetic d) Ar is diamagnetic e) I is paramagnetic f) a through e are all true g) a through e are ... Argon Orbital diagram, Electron configuration, and Valence ... S orbital contain 1 box that can hold a maximum of 2 electrons. P orbital contains 3 boxes that can hold a maximum of 6 electrons. D orbital contains 5 boxes that can hold a maximum of 10 electrons. F orbital contains 7 boxes that can hold a maximum of 14 electrons. The orbital diagram will also be filled with the same order as described by the ... What Is Used To Represent An Orbital In An Orbital ... Electron Configuration Standard Notation The number and letter describe the energy level and orbital, and the number above the orbital shows how many electrons are in that orbital. Using standard notation, the electron configuration of fluorine is 1s22s22p5. What do the arrows in orbital notation represent? An orbital diagram, or orbital box ... What is the maximum number of electrons that can occupy a ... The major difference between a 1s orbital and a 2s orbital is that; The electrons that occupy the outermost filled shell are called; When filling degenerate orbitals, electrons fill them singly first, with parallel spins is known as; You can click the format cells dialog box launcher to open the format cells dialog box.





























0 Response to "44 what is the maximum number of electrons that can occupy a box in an orbital filling diagram"
Post a Comment