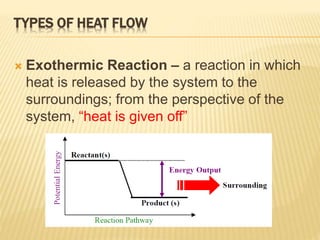
42 exothermic potential energy diagram
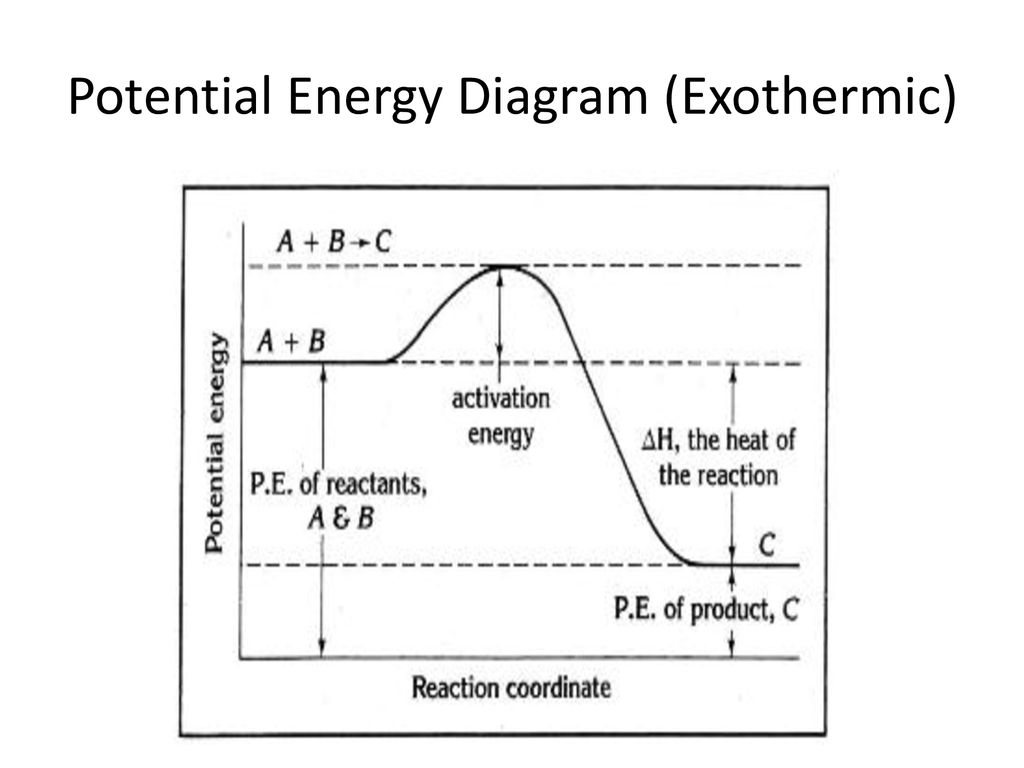
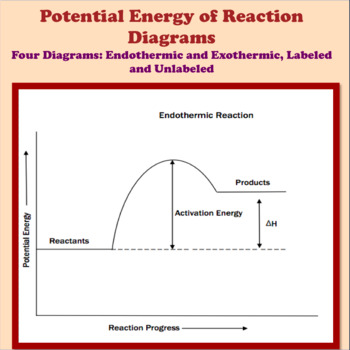
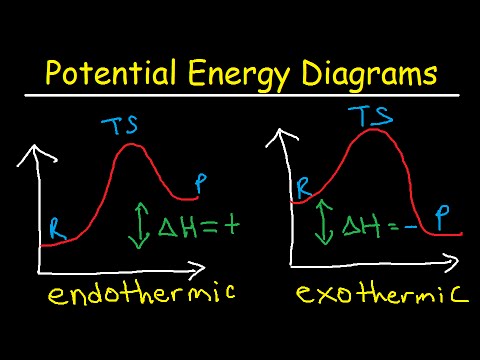
Endothermic and Exothermic Potential Energy Diagrams The fall in the graph represents the heat being released and the energy being lost Endothermic and Exothermic Potential Energy Diagrams Diagrams (+q) products ΔH is positive reactants (-q). When heat flows into an exothermic reaction it releases heat and energy is lost from the system. How to draw the potential energy diagram for this reaction? | Socratic 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants...
Exothermic Potential Energy Diagram Labeled - Diagram Media 5 potential energy diagram exothermic rxn potential energy diagram labeled photoshots diagrom fine endothermic reactions with medium The diagram represents the potential energy changes when a cold pack is activated. Energy profile diagrams for endothermic and exothermic reactions.
Exothermic potential energy diagram
Chemistry 30 Chemical Kinetics - Potential Energy Diagrams Revisited 3.2 Potential Energy Diagrams Revisited. In our unit on Thermochemistry the terms endothermic and exothermic were discussed. Endothermic reactions require a net input of energy. Exothermic reactions release energy to the surroundings. Potential Energy Diagrams. Eilisha Joy Bryson If there is more energy released when the bonds form between the products compared to the amount of energy absorbed to break the bonds between reactants, then the In class we analyzed ATP and learned that ATP hydrolysis, ATP + H2O ® ADP + P, is an exothermic reaction, the water breaks the... Issue 33: Chemistry: Exothermic/Endothermic (Hoyle's Freshman...) Potential Energy Diagram. If reactants are higher than products, the reaction is exothermic. ΔH=potential energy of products-potential energy of reactants. Universe favors lower energy systems. Activation energy-energy needed to get a reaction started.
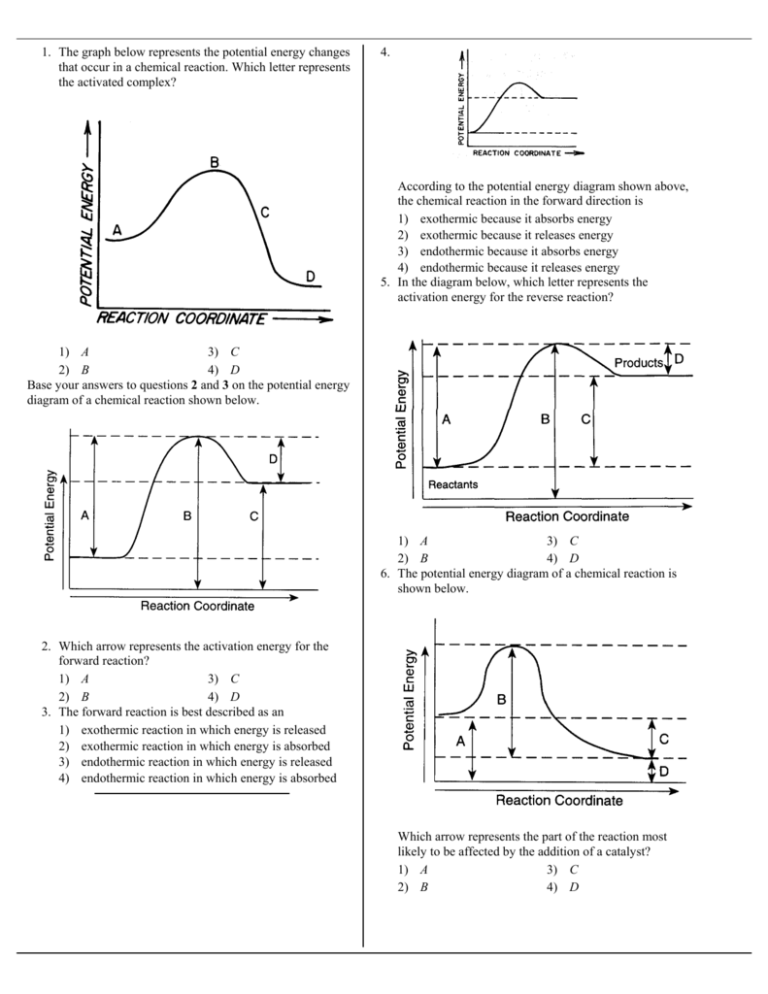
Exothermic potential energy diagram. 1. which type of chemical reaction results in the absorption ... Feb 03, 2022 · 1. which type of chemical reaction results in the absorption of energy? a.exothermic b.combustion c.replacement d.endothermic 2. which type of chemical reaction occurs when natural gas is burned? 1. replacement 2. endothermic 3. decomposition 4. exothermic 3. which part of an energy graph is represented by the highest point? 1. the products of the reaction 2. the activated state 3. the amount ... Potential Energy Diagrams - Chemistry - Catalyst, Endothermic... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the... Potential Energy Diagram Exothermic Potential Energy Diagrams Endothermic Potential Energy Diagrams How to read Potential Energy Diagrams. you will know about the Potential Energy Changes takes place during the formation of Hydrogen molecule.this topic is short ... Exothermic Reaction - Definition and Examples | Properties ... Exothermic Reactions is the flow of the net transfer of heat energy during the reaction is from the medium into its surroundings. In exothermic reactions, the reactants always possess more energy than the products and hence are less stable. For this reason, the exothermic reactions require very less amount of activation energy to initiate the reaction.
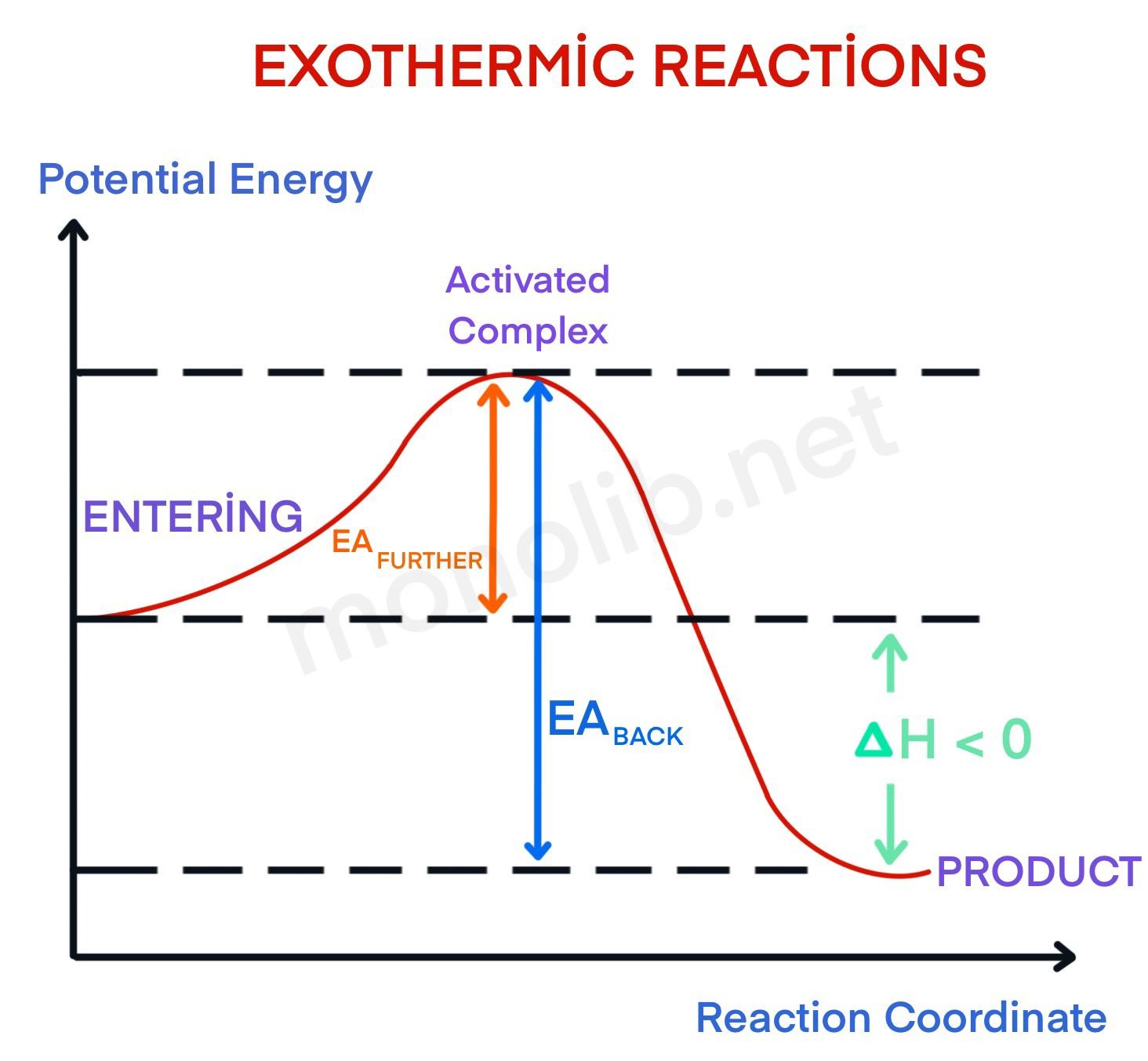
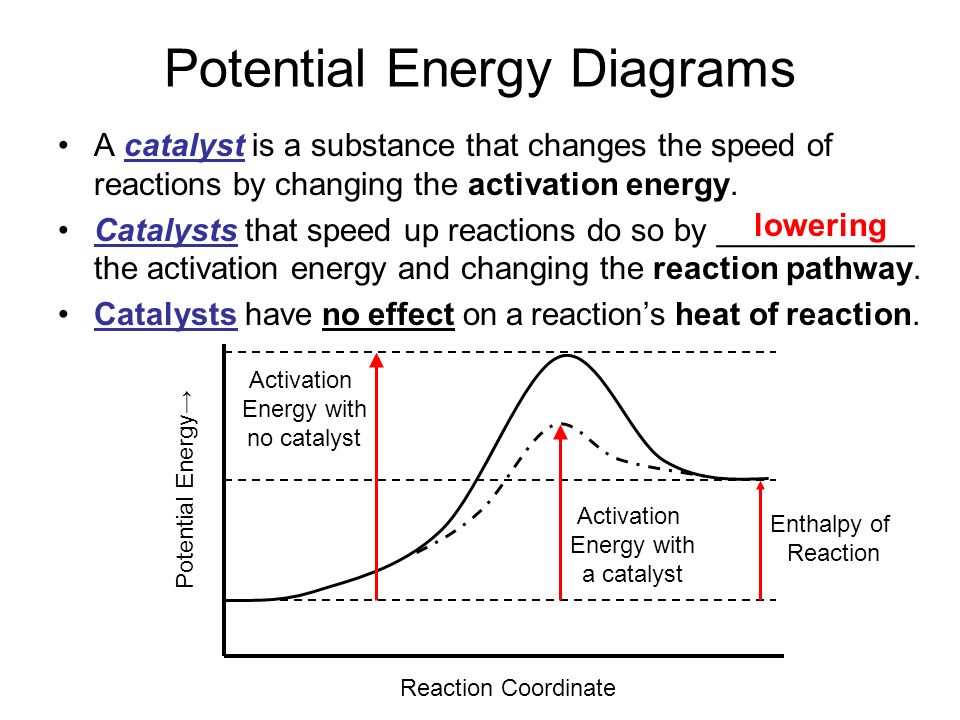
PPT Kinetics Lesson Potential Energy Diagrams Kinetic Energy... Simulation Potential Energy or Enthalpy (H). ΔH means change in enthalpy It is also called the heat of the reaction because it tells you how much heat or When PE (bond energy) decreases it is converted into KE which increases. Remember that KE is heat energy, so it gets hotter and it is exothermic. Diagram of Exothermic Potential Energy Diagram | Quizlet Start studying Exothermic Potential Energy Diagram. Learn vocabulary, terms and more with flashcards, games and other study tools. Energy & Heat | Exothermic vs Endothermic Reactions | Medium Potential and Kinetic Energy, and Types of Energy. Exothermic vs Endothermic Reactions, Exothermic Reaction Examples, and Endothermic Reaction Energy = the capacity to do work (w) or to produce heat (q). The concept of "energy" is familiar to us, but energy is a bit difficult to define. Potential Energy Diagrams Made Easy Exothermic followed by... 3 In any exothermic reaction, energy would be a product The potential energy of the reactants, labeled R, is steady, because it is based 23 Potential energy diagrams show the energy flow of a chemical reaction, from the starting point with the reactants, through the energy "spark" that will start...
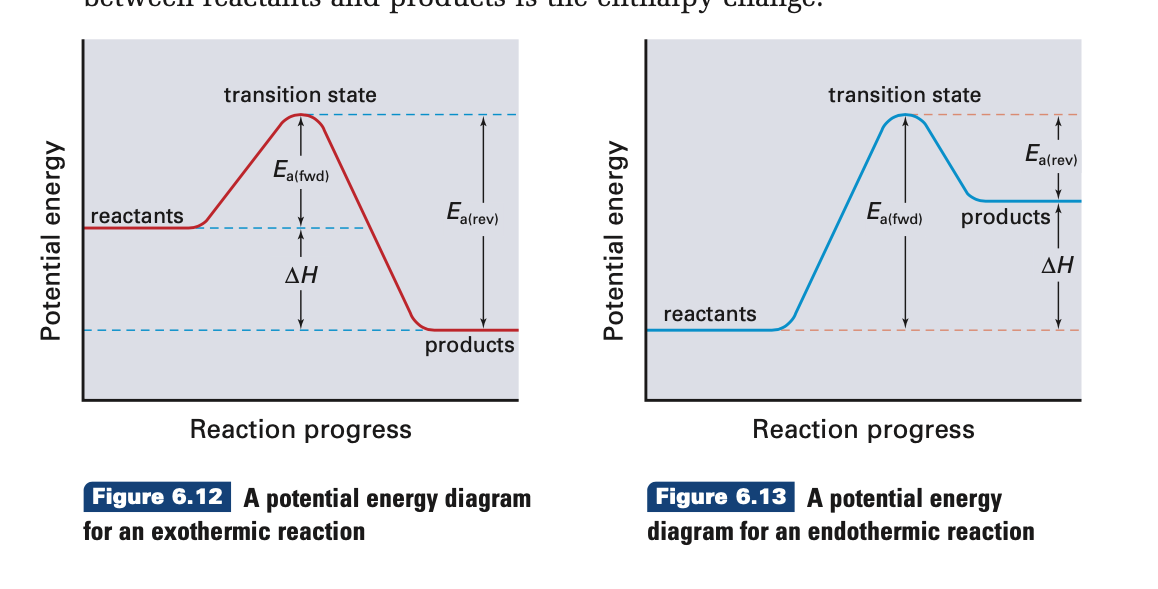
Gibbs Free Energy - Purdue University Reactions are classified as either exothermic (H < 0) or endothermic (H > 0) on the basis of whether they give off or absorb heat. Reactions can also be classified as exergonic (G < 0) or endergonic (G > 0) on the basis of whether the free energy of the system decreases or increases during the reaction.. When a reaction is favored by both enthalpy (H o < 0) and entropy (S o > … PDF Introduction to potential energy surfaces Diatomic potential energy curves To understand what a potential energy surface is, it is useful to start with something we have all seen - the potential energy curve for a diatom: Figure 1: This is Energy vs. r(AB) (distance between A and B). Where are re (equilibrium bond length), D0 (dissociation energy... What are Endothermic Reactions? (with Examples & Video) Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of … 13.5-13.6: Potential Energy Diagrams-Arrhenius Equation Potential Energy Diagrams: Example: *Endothermic Reaction. Ea = Activation Energy, the minimum energy of collision required for two In this example, the energy of the reactants is higher than that of the products, so heat energy is released when the reaction progresses, and the reaction is exothermic.
Exothermic - Potential Energy Diagram Chemistry 30 Chemical Kinetics - Potential Energy Diagrams Revisited. Exothermic - Potential Energy Diagram. Prairie South Schools 210. 24 followers. More information.
Dec 13, 2013 - Posts about IB Chemistry written by marvinamin Diagram. Activities. Chemistry 30 Chemical Kinetics - Potential Energy Diagrams Revisited. Exothermic - Potential Energy Diagram. Lily Sue. STEM - Physical Science.
PPT - Potential Energy Diagrams Made Easy Exothermic followed... In any exothermic reaction, energy would be a product. Potential energy in kJ/mol. time. Potential energy diagrams show the energy flow of a chemical reaction, from the starting point with the reactants, through the energy "spark" that will start a reaction, through the formation of the products...
Potential energy - Wikipedia In physics, potential energy is the energy held by an object because of its position relative to other objects, stresses within itself, its electric charge, or other factors. Common types of potential energy include the gravitational potential energy of an object that depends on its mass and its distance from...
How to know a chemical equation is exothermic or... - Quora If the energy diagram is not available, the sign of the energy value signifying the kind of the chemical If the energy is negative implies that the equation is exothermic equation but if its positive means Draw the graph between potential energy and progress of reaction for endothermic and...
Petroleum and Equations Jeopardy Template Draw a potential energy diagram for the digestion of a potato chip in your body. When you digest a potato chip, you are releasing energy stored in the bonds of the potato (and the grease!!), so it is exothermic. A diagram showing the lowering of the energy should be drawn...
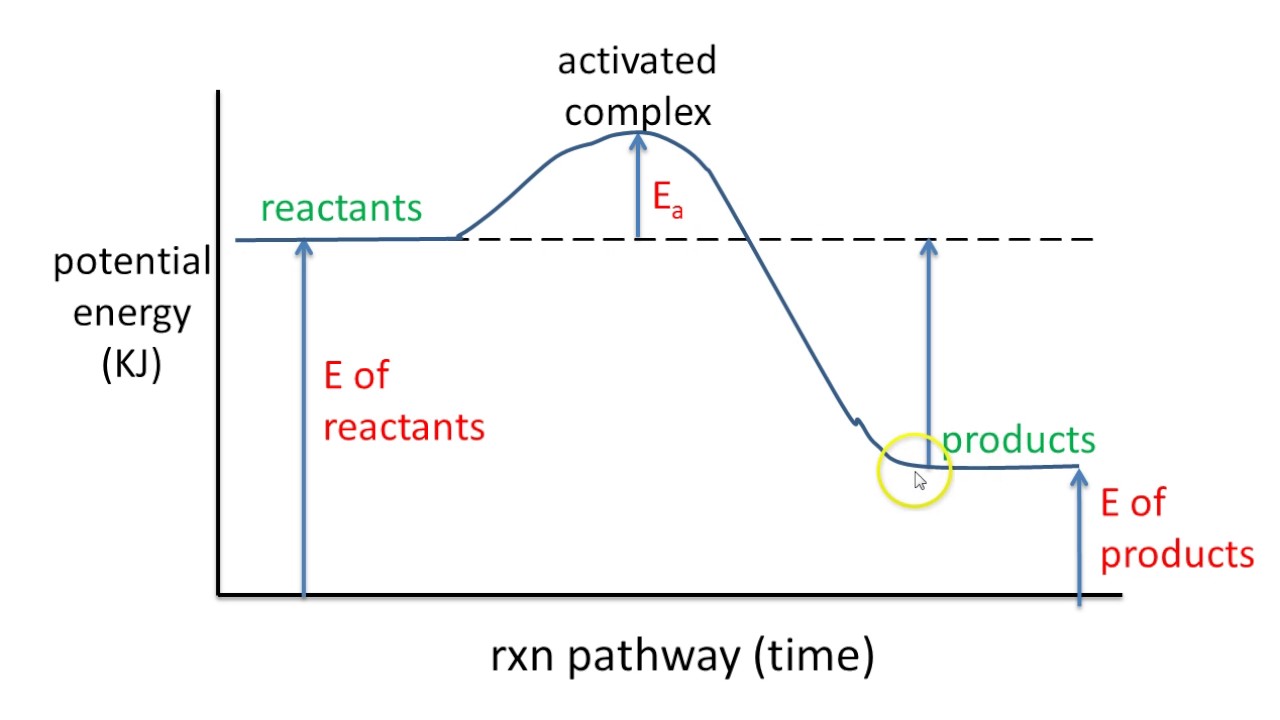
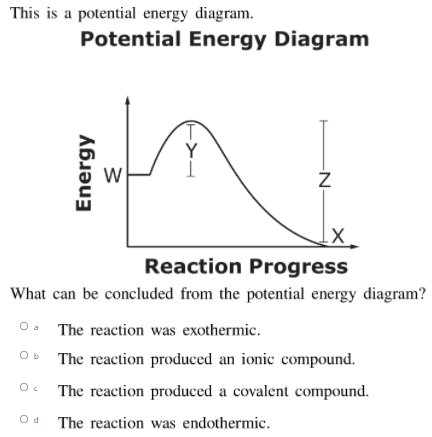
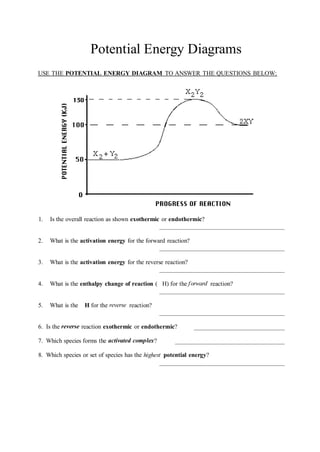
Potential Energy Diagrams - Kentchemistry.com A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values.
How to tell if a reaction is exothermic or endothermic from ... Image of a graph showing potential energy in relation to the process of a chemical reaction. In the case of an exothermic reaction, the reactants are at a higher energy level as compared to the products, as shown below in the energy diagram. In other words, the products are more stable than the reactants.
equal and opposite energy quick check A researcher can ... Feb 01, 2022 · relative potential energy 2 quick check. 1. which type of chemical reaction results in the absorption of energy? a.exothermic b.combustion c.replacement d.endothermic 2. which type of chemical reaction occurs when natural gas is burned? 1. replacement 2. endothermic 3. science
Exothermic Potential Energy Diagram - Free Catalogs A to Z 18.4: Potential Energy Diagrams - Chemistry LibreTexts. 1 hours ago A potential energy diagram shows the change in potential energy of a system 8 hours ago Decrease in kinetic energy - energy released - exothermic Energy level diagrams Chemical Potential Energy The chemical potential...
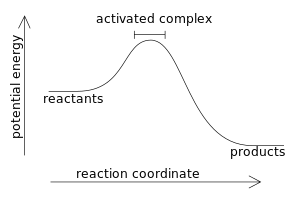
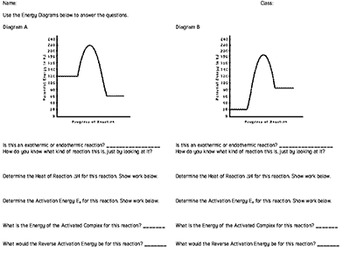
Potential energy diagrams - Controlling the rate - Higher Chemistry... Potential energy diagrams can be used to calculate both the enthalpy change and the activation energy for a reaction. Exothermic reactions. The activated complex (high energy intermediate state where bonds are breaking and forming) can be shown on potential energy diagrams.
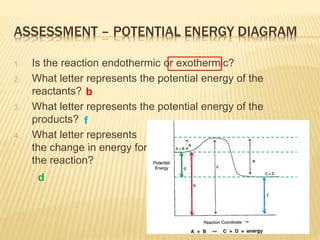
Chem 30 - 4.3 - Energy Diagrams - Practice with Key - chem30-wmci Sketch a potential energy diagram for a general reaction A + B Given that ΔHreverse = -10 kJ and Ea forward = +40 kJ Solution: Begin by determining whether the forward reaction (A + B → C + D) is endothermic or exothermic: Since ΔHreverse = -10 kJ you can determine that ΔHforward = +10 kJ...
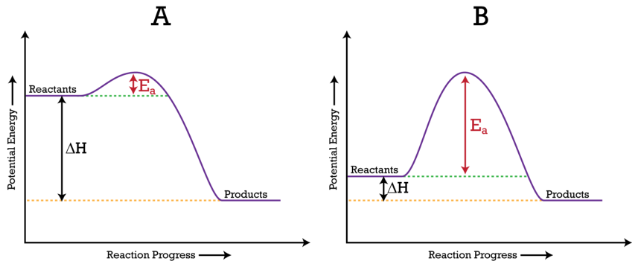
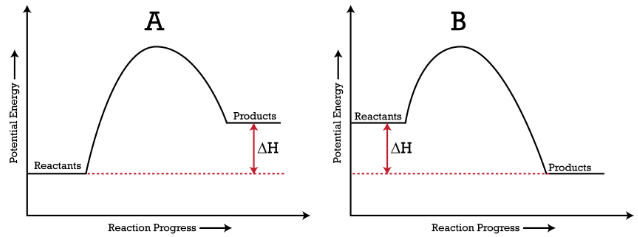
18.4: Potential Energy Diagrams - Chemistry LibreTexts A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change [Math Processing Error].
Potential energy diagram for the decomposition of potassium chlorate charcoal, combustion, activation energy, exothermic, potential energy. Activation energy diagram with forward path and reverse paths. Caption. For the metabolism or oxidation of glucose, the reaction path is forward, over the small activation energy hill.

Draw the potential energy diagram for an exothermic reaction. Explain the terms: activation energy of forward reaction
Exothermic and Endothermic Processes | Introduction to Chemistry Exothermic reactionIn an exothermic reaction, the total energy of the products is less than the total energy of the reactants. An energy diagram can be used to show energy movements in these reactions and temperature can be used to measure them macroscopically.
Examples of Chemical Energy in Everyday LIfe 9 Examples of Chemical Energy. Chemical energy is either released (exothermic reaction) or absorbed (endothermic reaction) during a chemical reaction. In an exothermic reaction, heat is released, creating warmth. In an endothermic reaction, the heat is absorbed, creating cooling. Air bags are activated by a chemical reaction inside the bag. A ...
PDF Potential Energy Surfaces A potential energy surface is a mathematical function that gives the energy of a molecule as a function of its geometry. • Molecular Mechanics provides this energy as a function of stretches, bends, torsions, etc. This is an approximate model that breaks down in some situations (e.g., breaking bonds).
What is Enthalpy? - Definition, Endothermic & Exothermic ... Enthalpy diagram. When a process begins at constant pressure, the evolved heat (either absorbed or released) equals the change in enthalpy. Enthalpy change is the sum of internal energy denoted by U and product of volume and Pressure, denoted by PV, expressed in the following manner. H=U+PV. Enthalpy is also described as a state function completely based on …
Exothermic Reaction: Energy Diagram - Bio Differences Exothermic Reaction: Energy Diagram. In an exothermic system, the graph plotted between stored potential energy and the progress of reaction: This graph clearly displays that the reactants lie at a higher energy point in comparison to the product.
Potential Energy Diagrams ( Read ) | Chemistry | CK-12 Foundation Explains potential energy diagrams and activation energy. Learning Objectives. Vocabulary. Search Keywords: activation energy endothermic reaction exothermic reaction (1 more) potential energy diagram.
Energy Conservation Quick Check 1.Is freezing an ... 15/02/2022 · Energy Conservation Quick Check 1.Is freezing an endothermic or exothermic process? How do you know?(1 point) Freezing is exothermic because as water bonds into ice, the bonds absorb energy from the environment in order to change states. Freezing is exothermic because as water bonds into ice, the energy from bond formation is released and heats up the …
Potential diagrams - Big Chemical Encyclopedia Exothermic reaction potential energy diagram. Potential Energy Diagrams for Multistep Reactions The SN1 Mechanism. Potential energy Diagram and Kinetic Parameters.
Issue 33: Chemistry: Exothermic/Endothermic (Hoyle's Freshman...) Potential Energy Diagram. If reactants are higher than products, the reaction is exothermic. ΔH=potential energy of products-potential energy of reactants. Universe favors lower energy systems. Activation energy-energy needed to get a reaction started.
Eilisha Joy Bryson If there is more energy released when the bonds form between the products compared to the amount of energy absorbed to break the bonds between reactants, then the In class we analyzed ATP and learned that ATP hydrolysis, ATP + H2O ® ADP + P, is an exothermic reaction, the water breaks the...
Chemistry 30 Chemical Kinetics - Potential Energy Diagrams Revisited 3.2 Potential Energy Diagrams Revisited. In our unit on Thermochemistry the terms endothermic and exothermic were discussed. Endothermic reactions require a net input of energy. Exothermic reactions release energy to the surroundings. Potential Energy Diagrams.


















0 Response to "42 exothermic potential energy diagram"
Post a Comment