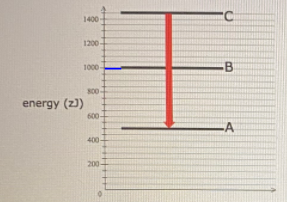
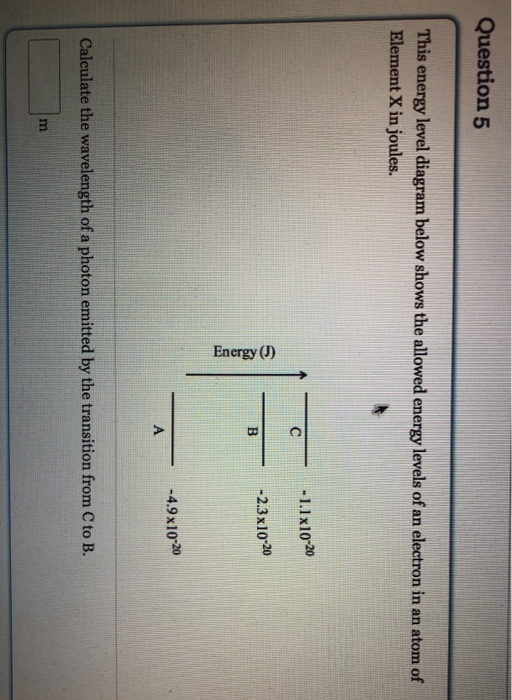
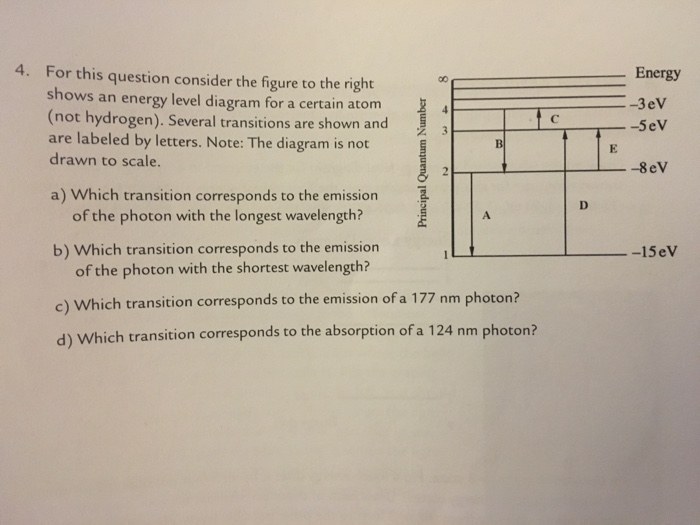
42 for this question consider the figure to the right shows an energy level diagram for a certain atom
In the pharmacy, it is preferred to use the metric measuring Related Questions. An experiment is conducted in which red light is diffracted through a single slit; You hold a wire coil so that the plane of the coil is perpendicular to a magnetic field B; For this question consider the Figure to the right shows an energy level diagram for a certain atom not hydrogen Energy level diagrams and the hydrogen atom Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states.
Solved For This question consider the figure to the right - Chegg Question: For This question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown ...
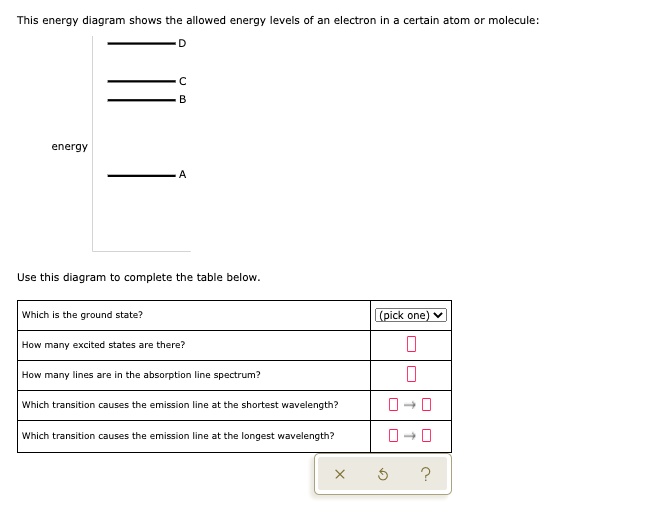
For this question consider the figure to the right shows an energy level diagram for a certain atom
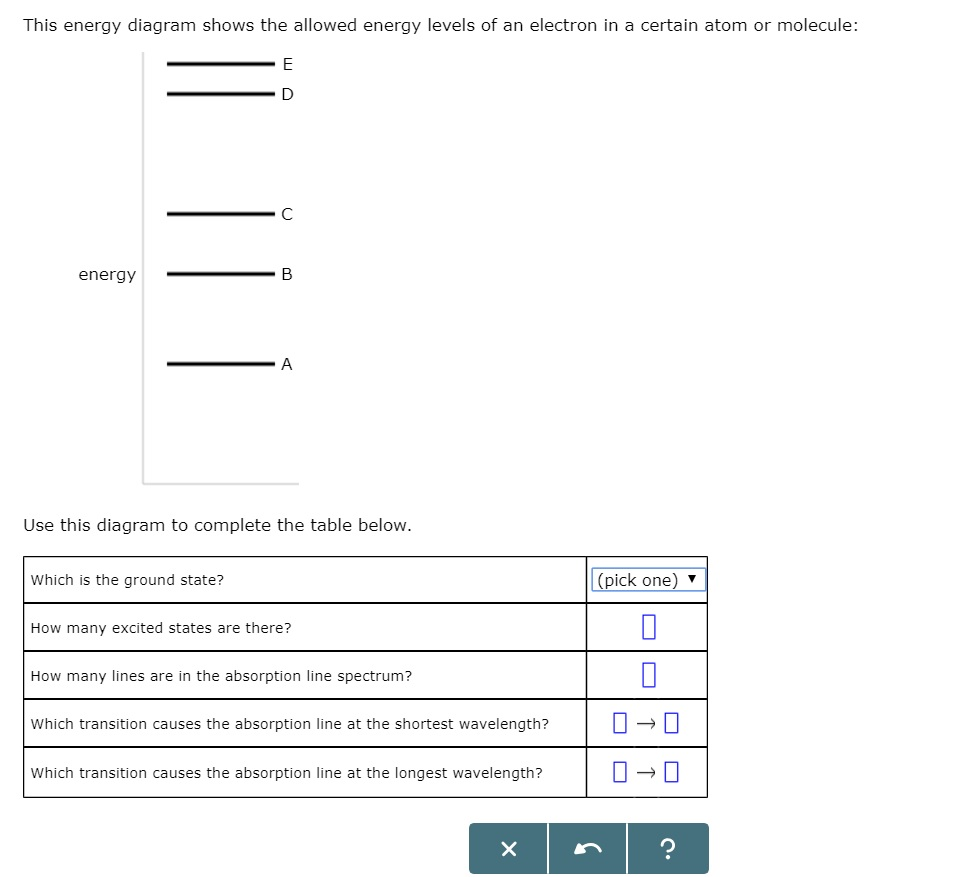
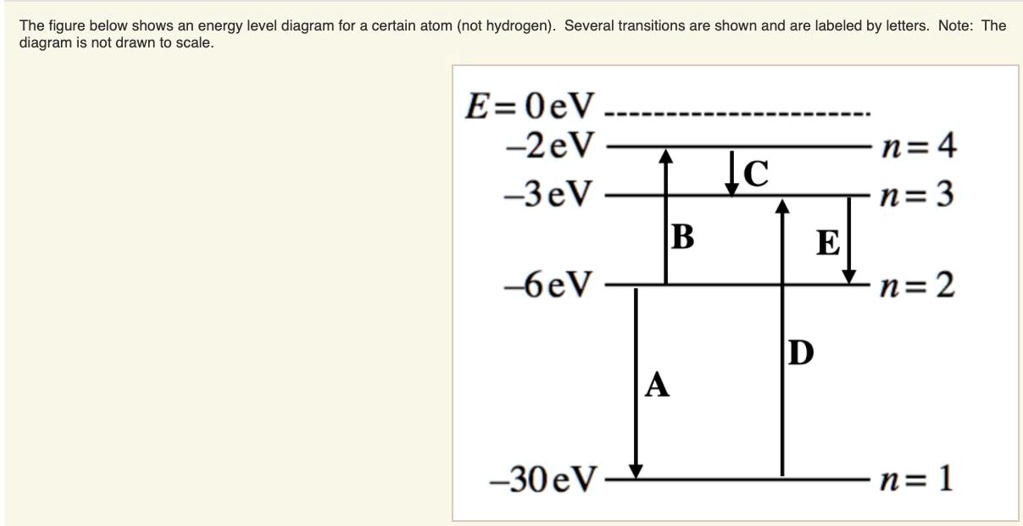
For this question consider the Figure to the right shows an For this question consider the Figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Chapter 5 Concept Quiz Flashcards | Quizlet B. You can tell because the electron jumps up from 0 eV to 10.2 eV. The diagram represents energy levels in a hydrogen atom. The labeled transitions (A through E) represent an electron moving between energy levels. Suppose that an electron in a hydrogen atom absorbs 10.2 eV of energy, so that it moves from level 1 to level 2. Solved For this question consider the Figure to the right - Chegg Question: For this question consider the Figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown ...
For this question consider the figure to the right shows an energy level diagram for a certain atom. Solved For this question consider the figure to the right ... Science Physics Physics questions and answers For this question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale. [Best Answer] The diagram shows Niels Bohr's model of an ... The diagram shows Niels Bohr's model of an atom. What happens when the electron moves from the first energy level to the second energy level? Energy is absorbed, and an emission line is produced. Energy is released, and an emission line is produced.[wrong] Energy is absorbed by the atom. Energy is lost from the atom. physics 28-31 Flashcards - Quizlet The figure shows an energy level diagram for the hydrogen atom. Several transitions are shown and are labeled by letters.Note: The diagram is not drawn to scale. Which transition corresponds to the absorption of the photon with the longest wavelength? (Get Answer) - a Explain how the emission lines in the ... Pls help! For this question consider the Figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale. Which transition...
Atomic Energy Levels (video) - Khan Academy We like representing these energy levels with an energy level diagram. The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV. (Get Answer) - The Lyman series in the hydrogen atom ... Pls help! For this question consider the Figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale. Which transition... The figure is an energy-level diagram for a simple atom. For this question consider the figure to the right that shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and labeled by letters. Note the diagram is not drawn to scale. (A) Which transition corresponds to the emission of the photon with the longest wavelength? Energy Level and Transition of Electrons | Brilliant Math ... In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. According to Bohr's theory, electrons of an atom revolve around the nucleus on certain orbits, or electron shells. Each orbit has its specific energy level, which is expressed as a negative value. This is because the electrons on the orbit are "captured ...
Energy Level Diagram For Hydrogen - Mini Physics The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV. When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon. Solved Energy For this question consider the figure to the Question: Energy For this question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen). For this question consider the figure that... | Clutch Prep For this question consider the figure that shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale. Which transition corresponds to the emission of the photon with the longest wavelength? Solved For this question consider the figure to the right ... Physics. Physics questions and answers. For this question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Which transition corresponds to the emission of the photon with the longest wavelength?
For this question consider the Figure to t... | Clutch Prep Problem: For this question consider the Figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labelled by letters. Note: The diagram is not drawn to scale. Which transition corresponds to the absorption of a 124 nm photon?
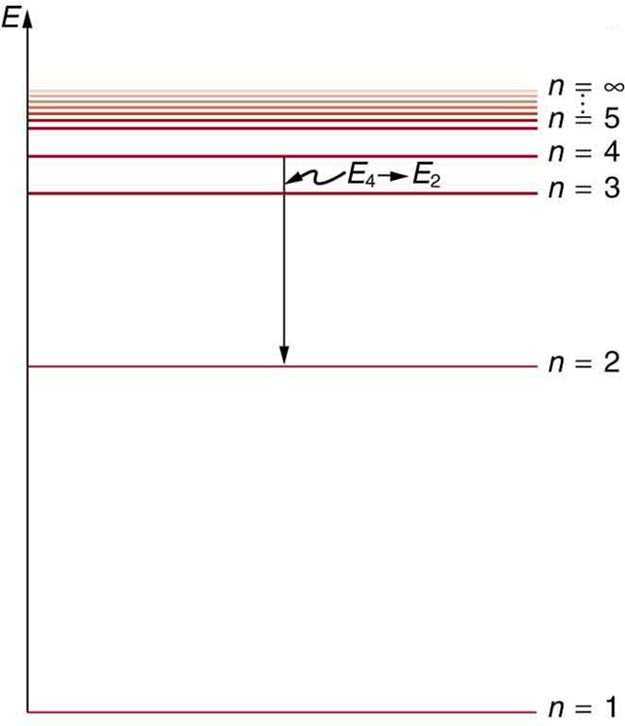
22.1 The Structure of the Atom - Physics | OpenStax Figure 22.10 An energy-level diagram plots energy vertically and is useful in visualizing the energy states of a system and the transitions between them. This diagram is for the hydrogen-atom electrons, showing a transition between two orbits having energies E 4 E 4 and E 2 E 2. The energy transition results in a Balmer series line in an ...
PDF 4. Energy Levels - MIT OpenCourseWare 4.3.1 The Hydrogen atom In the previous chapter we studied stationary problems in which the system is best described as a (time-independent) wave, "scattering" and "tunneling" (that is, showing variation on its intensity) because of obstacles given by changes
Solved For this question consider the figure to the right - Chegg Question: For this question consider the figure to the right shows an energy level diagram for a certain atom not hydrogen). Several transitions are shown ...
Chemistry midterm study guide Flashcards - Quizlet Using the sub-level diagram below, determine which sub-level is partially filled. ... T/F Valence refers to the total number of electrons that exist in the orbitals that occupy the outermost energy level of an atom. True. ... The difference between the atom on the left and the atom on the right is that the electron has been. Excited.
Energy Level of an Atom - Energy State and ... - VEDANTU The figure shows the energy levels of an atom. The first four energy levels are shown here. The first energy level is also called level 'K'. The second level is called level L, third energy level as M, and so on. The electrons from energy level K contains the least energy whereas the levels that are far from the nucleus contains more energy
Connect Assignment: Chapter 7 Electron Structure of the Atom 1. locate the position of the element on the periodic table. 2. find the closest noble gas in the period above the element. 3. write the symbol of the noble gas in brackets. 4. add the additional electron configuration needed to reach the element on the periodic table. select all the correct statements concerning valence electrons of elements.
Solution for Student Worksheet: Energy Levels in the Atom Solution for Calculate the Energy! Student Worksheet Neils Bohr numbered the energy levels (n) of hydrogen, with level 1 (n=1) being the ground state, level 2 being the first excited state, and so on.Remember that there is a maximum energy that each electron can have and still be part of its atom. Beyond that energy, the electron is no longer bound to the nucleus of the atom and it is ...
How to Represent Electrons in an Energy Level Diagram ... An energy level diagram is more useful and easier to work with than quantum numbers in the quantum mechanical model. Chemists use the energy level diagram as well as electron configuration notation to represent which energy level, subshell, and orbital are occupied by electrons in any particular atom.
Solved For this question consider the figure to the right - Chegg Transcribed image text: For this question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen).
PDF Waves review practice questions - The Leon M. Goldstein ... 46. The diagram shows two pulses, Aand B, moving to the right along a uniform rope. Compared to pulse A, pulse Bhas A. a slower speed and more energy B. a faster speed and less energy C. a faster speed and the same energy D. the same speed and more energy 47. A wave generator located 4.0 meters from a re ecting wall produces a standing wave in a
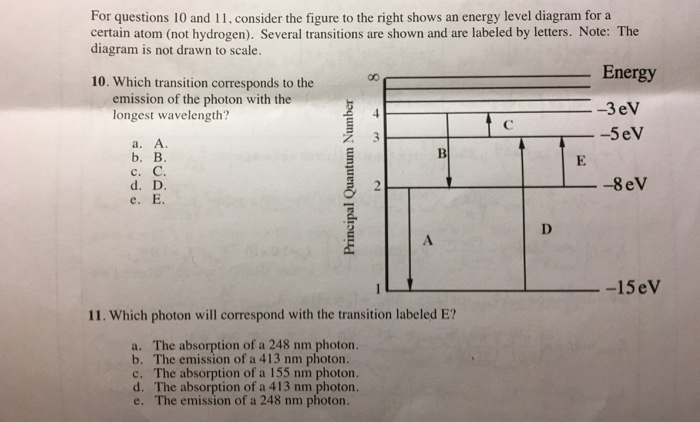
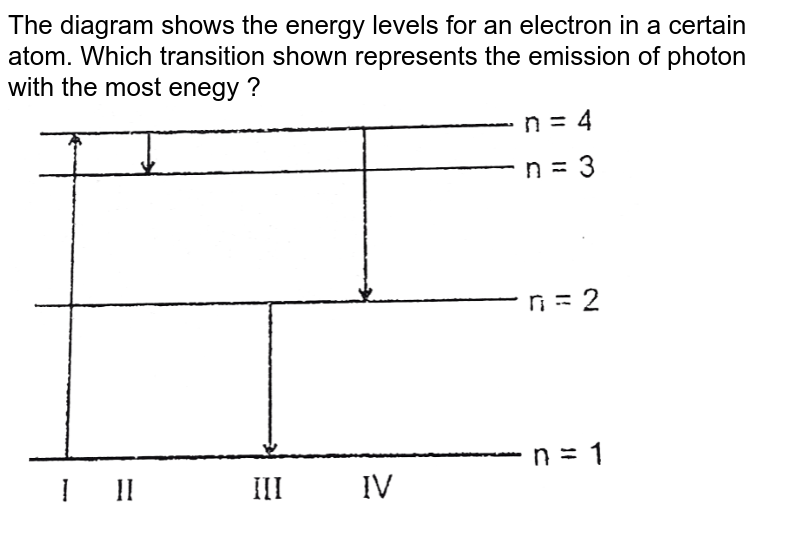
PDF ANSWER. Series #2 - LSU Figure 2 Refer to Figure 2 when answering questions #10 and #11. 10. In the two-level atom shown in Figure 2, which electron transition is associated with the emission of light? ANSWER. The transition labeled "b". 11. If the "c" transition marked in the three-level atom is associated with the absorption or
OneClass: The figure is an energy-level diagram for a ... For this question consider the figure to the right that shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and labeled by letters. Note the diagram is not drawn to scale. (A) Which transition corresponds to the emission of the photon with the longest wavelength?
Solved For questions 10 and 11, consider the figure to the Question: For questions 10 and 11, consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen).
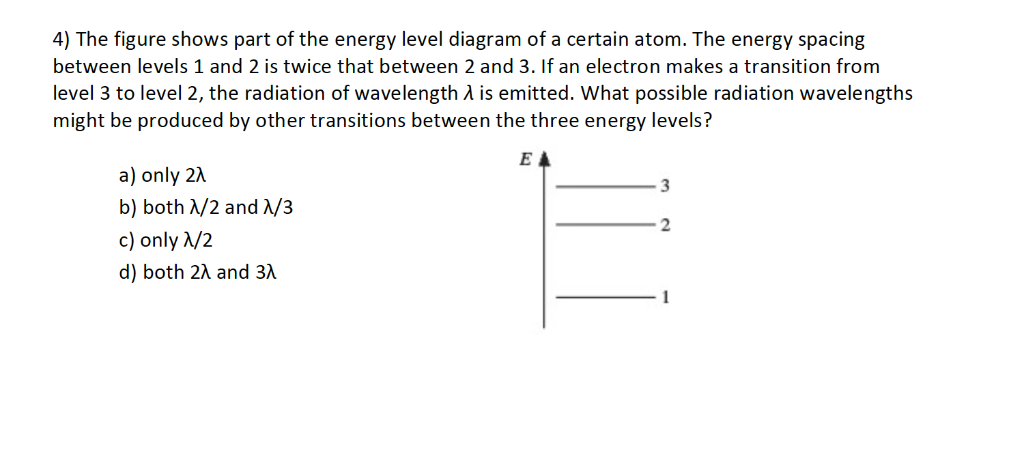
CH. 27 Flashcards - Quizlet The figure shows part of the energy level diagram of a certain atom. The energy spacing between levels 1 and 2 is twice that between 2 and 3. If an electron makes a transition from level 3 to level 2, the radiation of wavelength λ is emitted. What possible radiation wavelengths might be produced by other transitions between the three energy ...
Energy Level Diagram - Different Energy Shells Around the ... What is energy level diagram? In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. The closest shell to the nucleus is called the "K shell" followed by the "L shell" then the "M shell" and so on away from the nucleus.
Solved For this question consider the figure to the right ... Physics questions and answers; For this question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale. Which transition corresponds to the emission of the photon with the longest wavelength?
Solved ** PLEASE NOTE THIS QUESTION IS DIFFERENT FROM ... For this question consider the figure to the right shows an energy level diagram for a certain atom, (not hydrogen). Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale .
Solved For this question consider the Figure to the right - Chegg Question: For this question consider the Figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown ...
Chapter 5 Concept Quiz Flashcards | Quizlet B. You can tell because the electron jumps up from 0 eV to 10.2 eV. The diagram represents energy levels in a hydrogen atom. The labeled transitions (A through E) represent an electron moving between energy levels. Suppose that an electron in a hydrogen atom absorbs 10.2 eV of energy, so that it moves from level 1 to level 2.
For this question consider the Figure to the right shows an For this question consider the Figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters.



















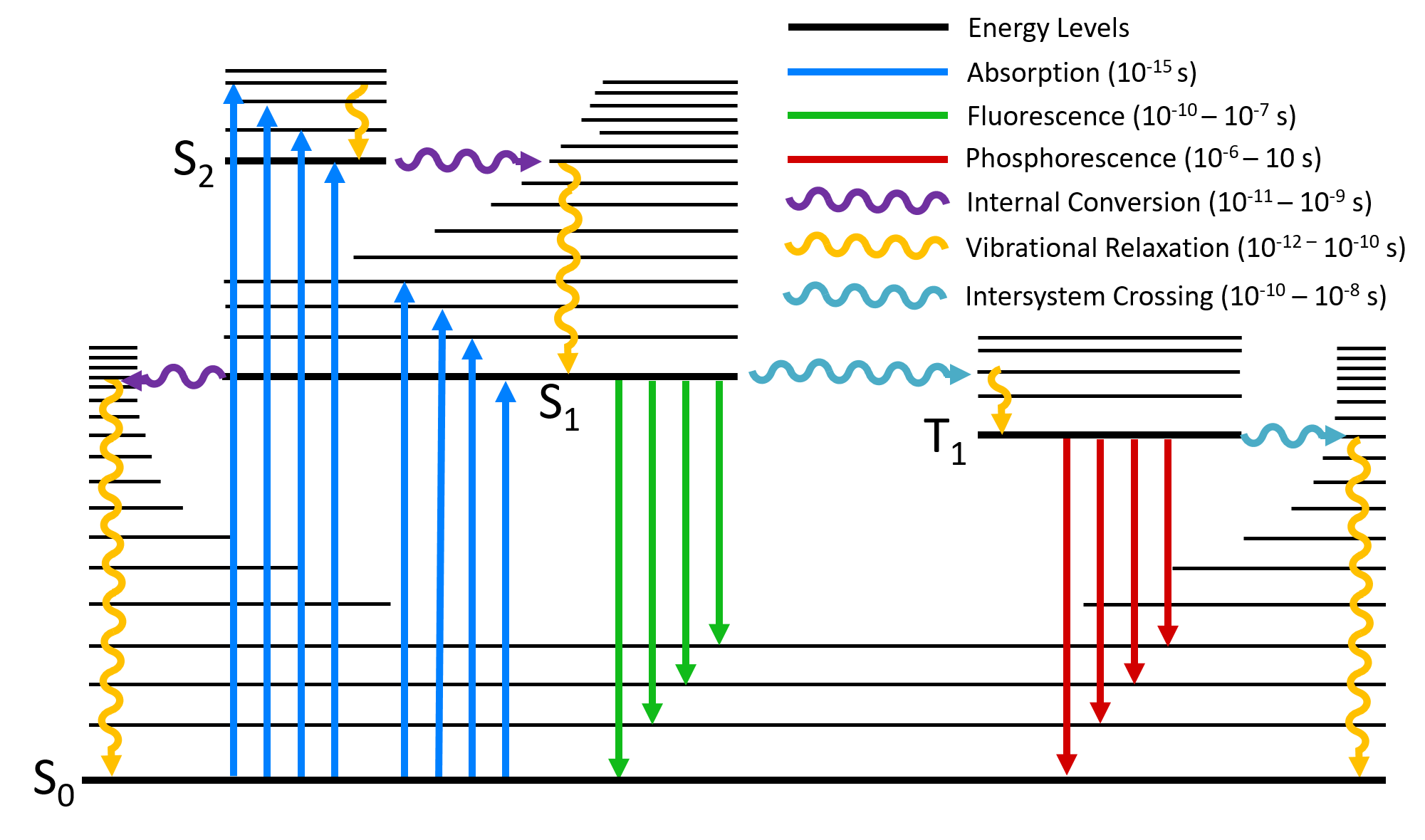


0 Response to "42 for this question consider the figure to the right shows an energy level diagram for a certain atom"
Post a Comment