42 octahedral molecular orbital diagram
Molecular orbital calculations for an octahedral cobalt ... Wolfsberg-Helmholtz molecular orbital calculations on an octahedral cluster of cobalt atoms have suggested that the 86 valence electrons in the carbonyl anion Co 6 (CO) 14 4- are all accommodated in bonding and weakly antibonding molecular orbitals and consequently represent a stable closed-shell electronic configuration. The 11 strongly antibonding skeletal molecular orbitals in such ... Lecture 3 Bonding Prof. Marcetta Y. Darensbourg myd@mail.chem.tamu.edu 408 Chemistry Bldg. (979) 845-5417 Office Hours: Th 11-12; M 11-12. Or any afternoon · Admin. Coord.: Abbey Kunkle darensbourg_asst@chem.tamu.edu 407 Chemistry Bldg. (979) 845-5417
PDF π-Bonding and Molecular Orbital Theory - Dalal Institute On the other hand, the bonding molecular orbitals of t2g are higher in energy than all σ bonding molecular orbitals. The overall molecular orbital energy level diagram for this type of π-bonding in octahedral complexes can be shown as: Figure 21. The generation of π and σ-molecular orbitals in octahedral complexes.
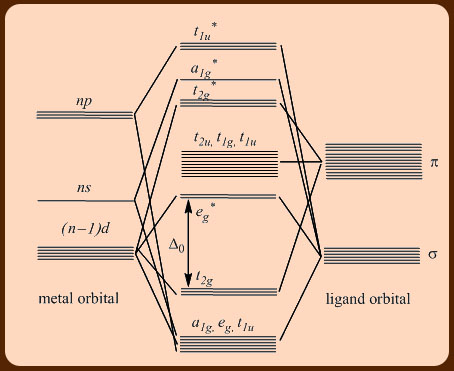
Octahedral molecular orbital diagram
Molecular Orbital Diagram of complexes@The Big Concept: PG ... In about 15 minutes animated video, the concept of Molecular Orbital Theory (MOT), symmetry of metal atomic orbitals in different ligand environment and mole... Molecular Orbital Diagram for Tetrahedral Compound ... Homework Statement. I'm trying to construct a molecular orbital correlation diagram for a tetrahedral compound [NiX 4] 2- considering the ligand pi-orbital basis. I've already constructed a diagram wherein the only the sigma-orbital basis is considered. In that diagram, I had 12 electrons for the nickel (10 in 3d - 6 with T 2 symmetry and 4 ... Molecular Orbital Theory for Octahedral and Tetrahedral ... Self‐consistent charge and configuration (SCCC) molecular orbital calculations are reported for 32 selected octahedral and tetrahedral first‐row transition‐metal complexes containing halide and chalcogenide ligands. It is found that for the range of metal oxidation states II through IV, F σ, chosen to fit the experimental Δ, is a function of only the metal atomic number for constant F π.
Octahedral molecular orbital diagram. Crystal Field Theory - Chemistry LibreTexts May 7, 2021 - Figure \(\PageIndex{5}\): (a) Tetraheral ligand field surrounding a central transition metal (blue sphere). (b) Splitting of the degenerate d-orbitals (without a ligand field) due to an octahedral ligand field (left diagram) and the tetrahedral field (right diagram). Metals, Tetrahedral and Octahedral - Chemistry LibreTexts September 16, 2020 - Thus, the electrons can fill the lowest energy molecular orbitals available to them. However, the electron pairing may be different if the electrons were allowed to fill the lowest energy atomic orbitals available to them. This diagram shows the field splitting of a metal with ligands in an octahedral ... PART 9(B): LIGAND FIELD THEORY (MO DIAGRAM OF OCTAHEDRAL COMPLEXES ... In this video I have explained SALC (Symmetry adopted linear combination) of transition metal valence orbitals and LGO'S (ligand group orbitals) to construct... Molecular Orbital Diagrams for Octahedral and Related ... Theoretical chemistry research group focusing on development of methods, and calculations in the areas of ionic liquids, photochemistry and catalysis
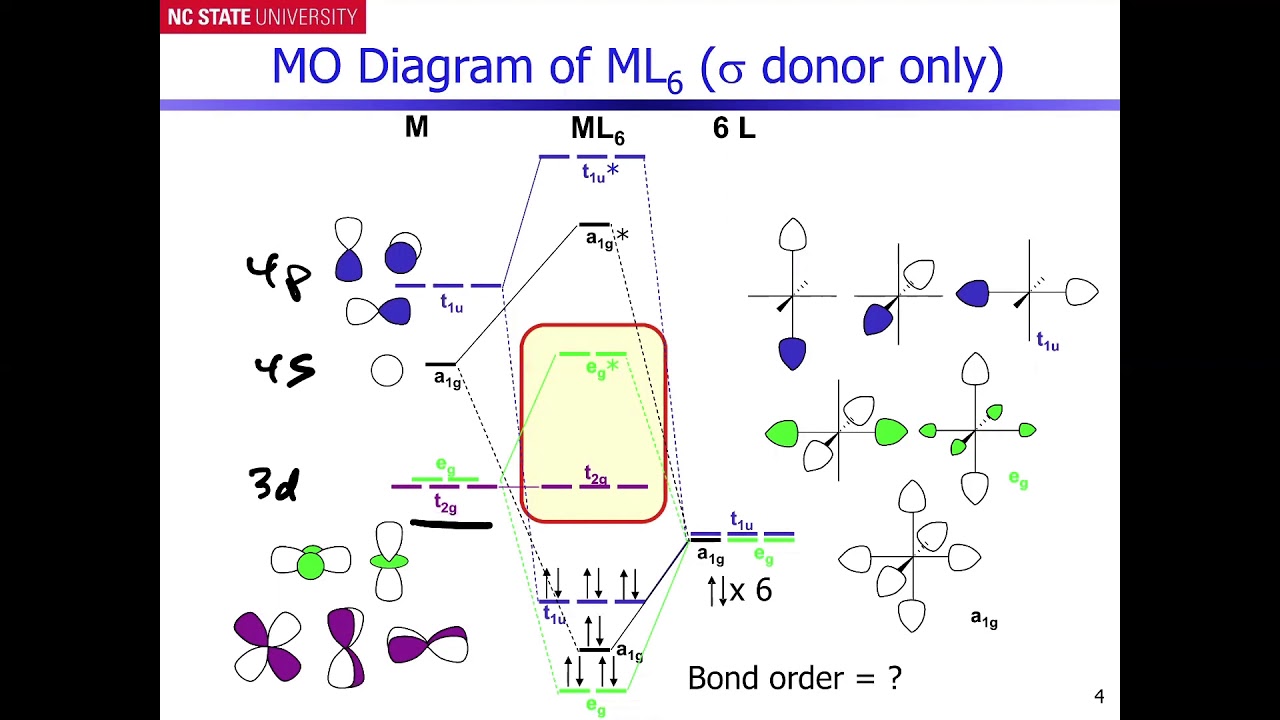
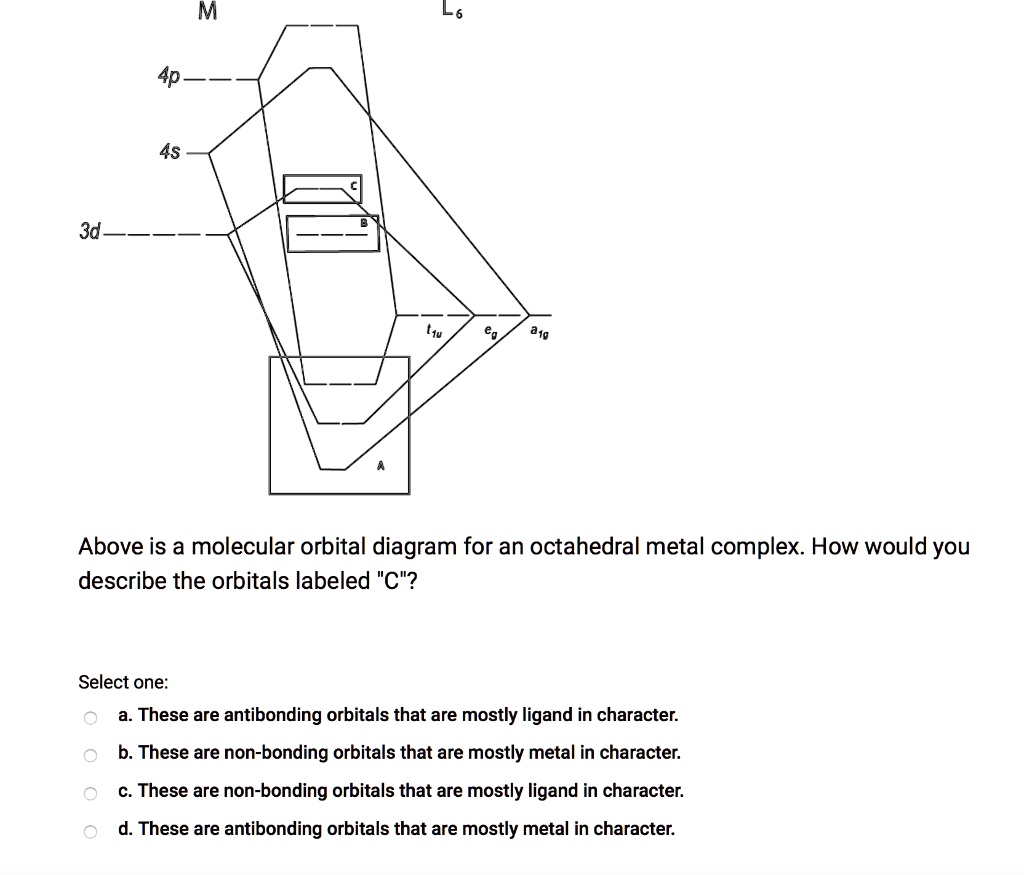
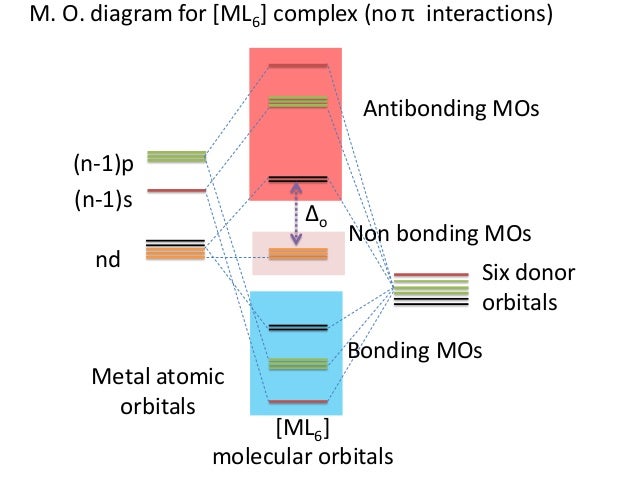
Qualitative valence molecular orbital (MO) diagram of an ... Qualitative valence molecular orbital (MO) diagram of an octahedral iron hexacyanide complex with Fe-centered nominal 3d and ligand-centered CN − MOs. The ferrous complex (Fe 2+ , 3d 6 ) has ... MOLECULAR ORBITAL THEORY AND BONDING NOTES The original version of this website (2003) was created back when I was at University, as a place to store my Chemistry notes for others - particularly later year groups - to have a little bit of help if they need it. I've recently (2019) brought the website up to date as it was showing serious ... d-Metal Complexes - University of Massachusetts Lowell A molecular orbital diagram which estimates the energies of the bonding (show above) antibonding and non-bonding orbitals is shown below. Since there is a large disparity in energy between the ligand orbitals and the metal orbitals, the lower lying molecular orbitals in the diagram are essentially ligand orbitals. DOC Molecular Orbital Theory of Octahedral Complexes The orbitals responsible for -bonding are not shown. The energy scale is expanded relative to the -complex MO diagram. Determine which diagram is for the -donor and -acceptor cases. Label the MOs with the appropriate symmetry labels and the o. ... Molecular Orbital Theory of Octahedral Complexes ...
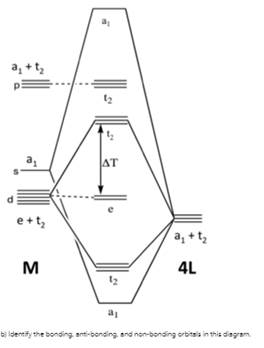
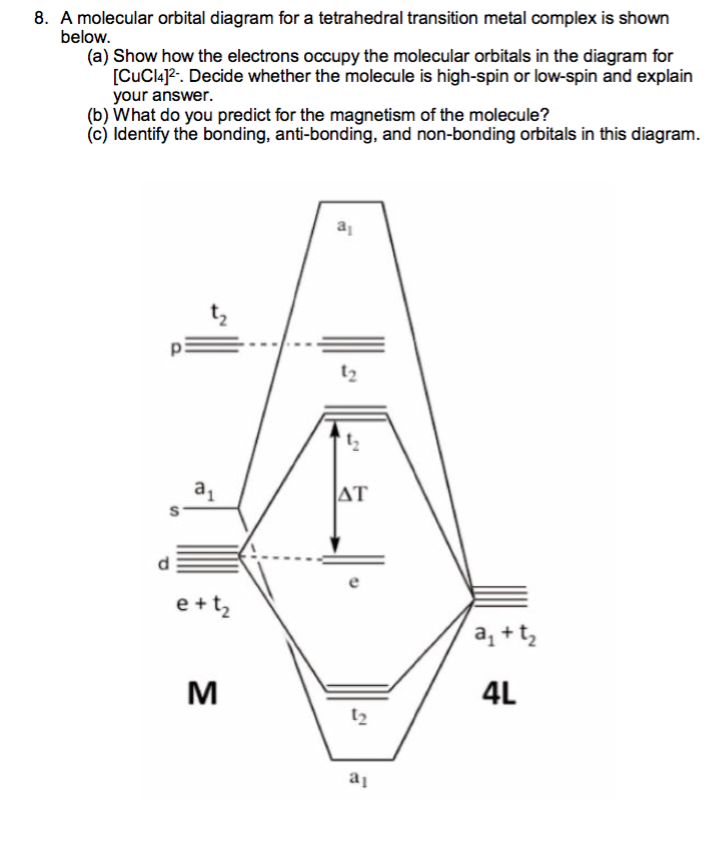
Octahedral molecular geometry - WikiMili, The Best ... For a free ion, e.g. gaseous Ni 2+ or Mo 0, the energy of the d-orbitals are equal in energy; that is, they are "degenerate". In an octahedral complex, this degeneracy is lifted. The energy of the d z 2 and d x 2 − y 2, the so-called e g set, which are aimed directly at the ligands are destabilized. On the other hand, the energy of the d xz, d xy, and d yz orbitals, the so-called t 2g set ... Molecular-orbital diagrams - Big Chemical Encyclopedia Simplified molecular orbital diagram for a low spia octahedral complex, such as [Co (NH3 )g, where A = energy difference a, e, and t may be antisymmetric (subscript ungerade) or centrosymmetric (subscript, gerade) symmetry orbitals. See text. Fig. 15.1 Molecular orbital diagram of inUamolecular donor (D) - chalcogen (E) interactions... PDF Chapter 10 Coordination Chemistry II: Bonding Molecular orbitals for Octahedral complexes The combination of the ligand and metal orbitals (4s, 4p x, 4p y, 4p z, 3d z2, and 3d x2-y2) form six bonding and six antibonding with a 1g, e g, t 1u symmetries. The metal T 2g orbitals do not have appropriate symmetry - nonbonding Electron in bonding orbitals provide the potential energy that holds ... handouts, Chem 1 :MO theoryh of complexes - Pomona College The molecular orbital diagram for a tetrahedral complex is given in Figure 3. The following features and consequences should be noted: The complex has 4 ligands and hence there are only 4 bonding molecular orbitals which are mostly ligand in character. These orbitals are completely filled as each ligand contributes as before a pair of electrons.
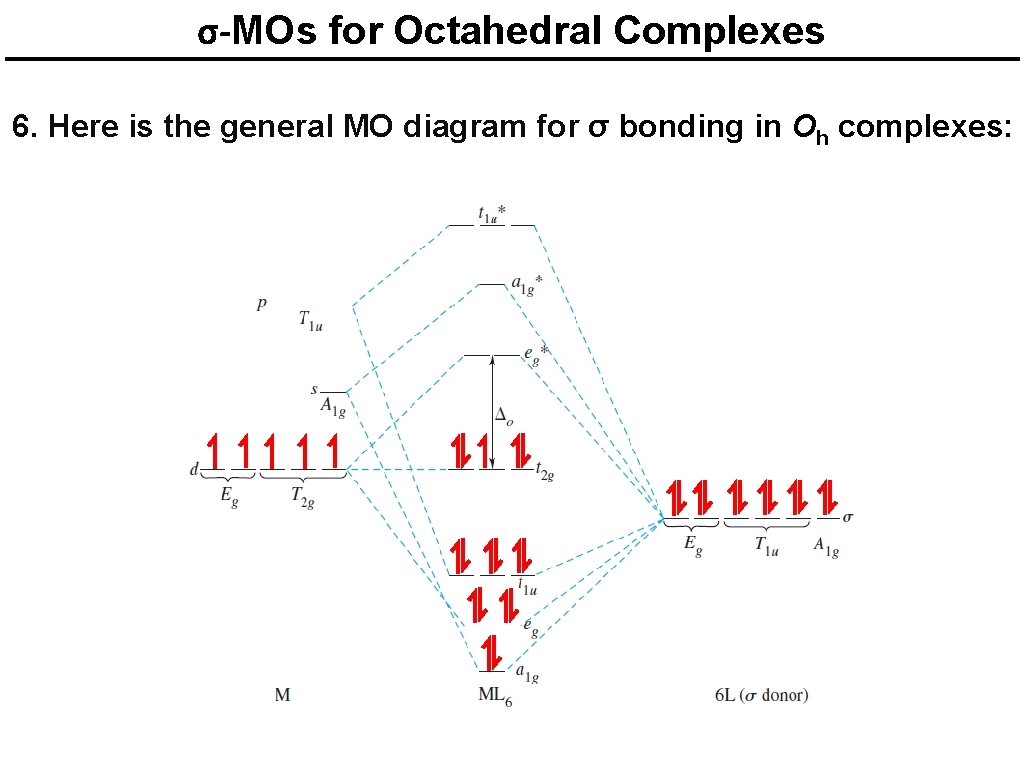
PDF MO Diagrams for More Complex Molecules d orbitals •l = 2, so there are 2l + 1 = 5 d-orbitals per shell, enough room for 10 electrons. •This is why there are 10 elements in each row of the d-block. σ‐MOs for Octahedral Complexes 1. Point group Oh 2. The six ligands can interact with the metal in a sigma or pi fashion. Let's consider only sigma interactions for now.
What is d orbital splitting in octahedral complex ... In octahedral symmetry the d-orbitals split into two sets with an energy difference, Δoct (the crystal-field splitting parameter, also commonly denoted by 10Dq for ten times the "differential of quanta") where the dxy, dxz and dyz orbitals will be lower in energy than the dz2 and dx2-y2, which will have higher energy.
Symmetry: episode 117, part 4 (molecular orbital diagrams of ... 00:21 Molecular orbital diagram for tetrahedral complex03:36 Molecular orbital diagram for octahedral complex"Kim Method" - MO diagrams for tetrahedral a...
7.1 Bonding in Coordination Compounds - Chemistry LibreTexts November 10, 2021 - Combine metal orbitals and ligand ... to form molecular orbitals. Select ligand orbitals for \(π\) and \(σ\) bonding if applicable, determine their symmetry and combine them with appropriate metal orbitals. Let us apply the ligand field theory to a 4th period octahedral transition ...
Ligand field theory - Wikipedia In an octahedral complex, the molecular orbitals created by coordination can be seen as resulting from the donation of two electrons by each of six σ-donor ligands to the d-orbitals on the metal. In octahedral complexes, ligands approach along the x-, y- and z-axes, so their σ-symmetry orbitals ...
Octahedral molecular geometry - Wikipedia Octahedral molecular geometry From Wikipedia, the free encyclopedia In chemistry, octahedral molecular geometry describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix octa.
PDF Molecular Orbital Theory - Octahedral, Tetrahedral or ... A total of six bonding and six anti-bonding molecular orbitals formed. The symmetry designations of different metal orbitals taking part in octahedral overlap are: dz2, dx2−y2- eg s - a1g px, py, pz- t1u dxy, dxz, dyz- t2g
Molecular orbital diagram corresponding to an octahedral ... Molecular orbital diagram corresponding to an octahedral MX N complex. Atomic levels of the transition-metal impurity, M, (ligand anions, X) are depicted on the left-hand (right-hand) side of the ...
Lecture 23 and 24_KRD New bifunctional TCNQ-based material · New bifunctional TCNQ based material: [Co(terpy)2](TCNQ)3·CH3CN exhibits a high room temperature conductivity of 0.13 S cm−1 and an anomaly in conductivity at ∼190 K as evidenced by variable temperature structural, magnetic and conductivity studies
Molecular orbital diagram, octahedral transition metal ... Fig. 11.53 Simplified molecular orbital diagram for an octahedral ML complex showing possible metal-to-ligand charge transfer (MLCT) transitions when both the t-ig and orbitals are occupied and the ligands have empty ir orbitals. Molecular orbital diagramsfor octahedral complexes, A.
Molecular orbital theory for octahedral complexes ... Molecular orbital theory Octahedral complexesDiagrams with examplesExplainedThanks for watching!!!!!#chemistry
BSc Chemistry - e-PG Pathshala Please use Internet Explorer web browser for site best experience or Click here · e-PG Pathshala is an initiative of the MHRD under its National Mission on Education through ICT (NME-ICT) being executed by the UGC. The content and its quality being the key component of education
Molecular Orbital Theory for Octahedral and Tetrahedral Metal ... November 22, 2019 - Basch, Harold and Viste, Arlen and Gray, Harry B. (1966) Molecular Orbital Theory for Octahedral and Tetrahedral Metal Complexes. Journal of Chemical Physics, 44 (1). pp. 10-19. ISSN 0021-9606. doi:10.1063/1.1726431. · Use this Persistent ...
Part One Structure and Bonding - Wiley-VCH
20.4: Molecular Orbital Theory - Octahedral Complexes - Chemistry ... February 3, 2021 - (Housecroft)/20%3A_d-Block_Metal_Chemistry_-_Coordination_Complexes/20.04%3A_Molecular_Orbital_Theory_-_Octahedral_Complexes
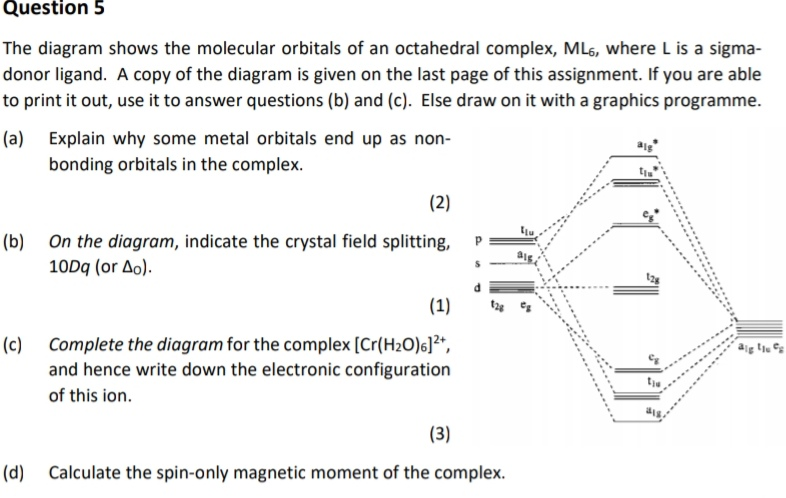
Solved Show below is a molecular orbital diagram for an ... Show below is a molecular orbital diagram for an octahedral ML6 complex and the Oh character table. From the ligand pi orbitals, only the t2g set is considered. All ligand orbitals are fully filled. Guiding lines connecting atomic orbitals to their corresponding molecular orbitals have been omitted.
From Atoms to Complexes – p. 1 - 6. Anorganic Molecules
Chemistry Glossary: Search results for 'octahedral ... This molecule is made up of six equally spaced sp 3 d 2 (or d 2 sp 3) hybrid orbitals arranged at 90° angles. The shape of the orbitals is octahedral. Two orbitals contain lone pairs of electrons on opposite sides of the central atom. The remaining four atoms connected to the central atom give the molecule a square planar shape.
Crystal field theory - Wikipedia The three lower-energy orbitals ... labels are based on the theory of molecular symmetry: they are the names of irreducible representations of the octahedral point group, Oh.(see the Oh character table) Typical orbital energy diagrams are given below in the section High-spin ...
6.3: Electronic Structure of Complexes (Part 2) - Chemistry ... August 14, 2020 - The characteristics of transition metal-ligand bonds become clear by an analysis of the molecular orbitals of a 3d metal coordinated by six identical ligands in octahedral complexes [ML6]. As the …
What is qualitative molecular orbital theory? - MSI In octahedral complexes, the molecular orbitals created by the coordination of metal center can be seen as resulting from the donation of two electrons by each of six σ-donor ligands to the d-orbitals on the metal. The metal orbitals taking part in this type of bonding are nd, (n+1)p and (n+1)s. It should be noted down
Molecular orbital theory of octahedral complexes Molecular orbital theory of octahedral complexes. 1. Molecular Orbital (M. O.) Theory (Octahedral Complexes) Dr. Mithil Fal Desai Shree Mallikarjun and Shri Chetan Manju Desai College Canacona Goa. 2. A σ M. O. for octahedral complex t2g, eg a1g, t1u, eg a1g t1u t* 1u a* 1g e* g eg a1g t1u Δo t2g Ligand group orbitalsMetal orbitals.
44 octahedral molecular orbital diagram - Modern Wiring ... An MO diagram is a descriptive instrument that is particularly used to explain the formation of chemical bonds in molecules with the help of molecular orbital theory. When atoms combine with other atoms to make molecules, some of the atomic orbitals adds up to form molecular orbitals which are the same in number.
Solved The molecular orbital diagram of an octahedral ... the molecular orbital diagram of an octahedral complex can be interpreted as containing the split d orbitals of crystal field with the qualification that the t2g and eg orbitals have the symmetry requirements of the hybrid atomic arbitals.tohyb the t2g and eg orbitals are bonding mos the t2g orbitals are nonbonding and the eg orbitals are …
Molecular Orbital Theory for Octahedral and Tetrahedral ... Self‐consistent charge and configuration (SCCC) molecular orbital calculations are reported for 32 selected octahedral and tetrahedral first‐row transition‐metal complexes containing halide and chalcogenide ligands. It is found that for the range of metal oxidation states II through IV, F σ, chosen to fit the experimental Δ, is a function of only the metal atomic number for constant F π.
Molecular Orbital Diagram for Tetrahedral Compound ... Homework Statement. I'm trying to construct a molecular orbital correlation diagram for a tetrahedral compound [NiX 4] 2- considering the ligand pi-orbital basis. I've already constructed a diagram wherein the only the sigma-orbital basis is considered. In that diagram, I had 12 electrons for the nickel (10 in 3d - 6 with T 2 symmetry and 4 ...
Molecular Orbital Diagram of complexes@The Big Concept: PG ... In about 15 minutes animated video, the concept of Molecular Orbital Theory (MOT), symmetry of metal atomic orbitals in different ligand environment and mole...









![Solved Based on molecular orbital (MO) diagram for [ML]](https://media.cheggcdn.com/study/06b/06bd1caa-a977-4fd5-8919-d972c571d8a4/image)



![d-orbital energy levels in planar [M II F 4 ] 2− , [M II (NH ...](https://pubs.rsc.org/image/article/2020/DT/d0dt02022b/d0dt02022b-f1_hi-res.gif)





0 Response to "42 octahedral molecular orbital diagram"
Post a Comment