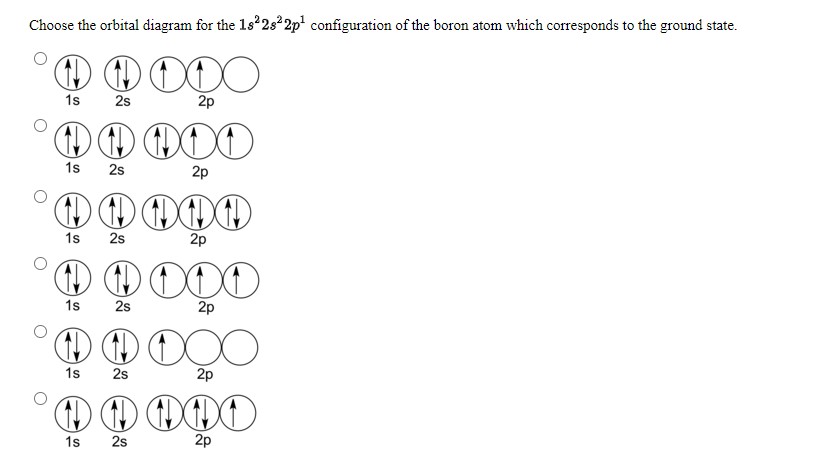
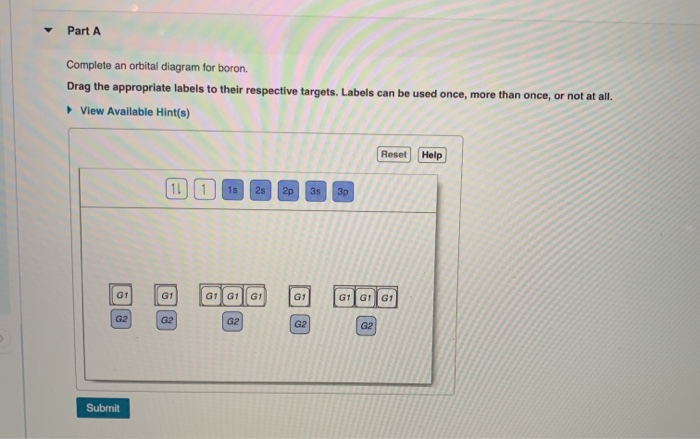
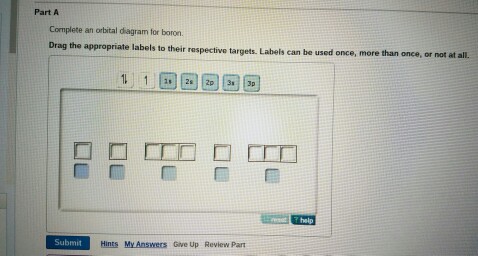
43 complete an orbital diagram for boron.
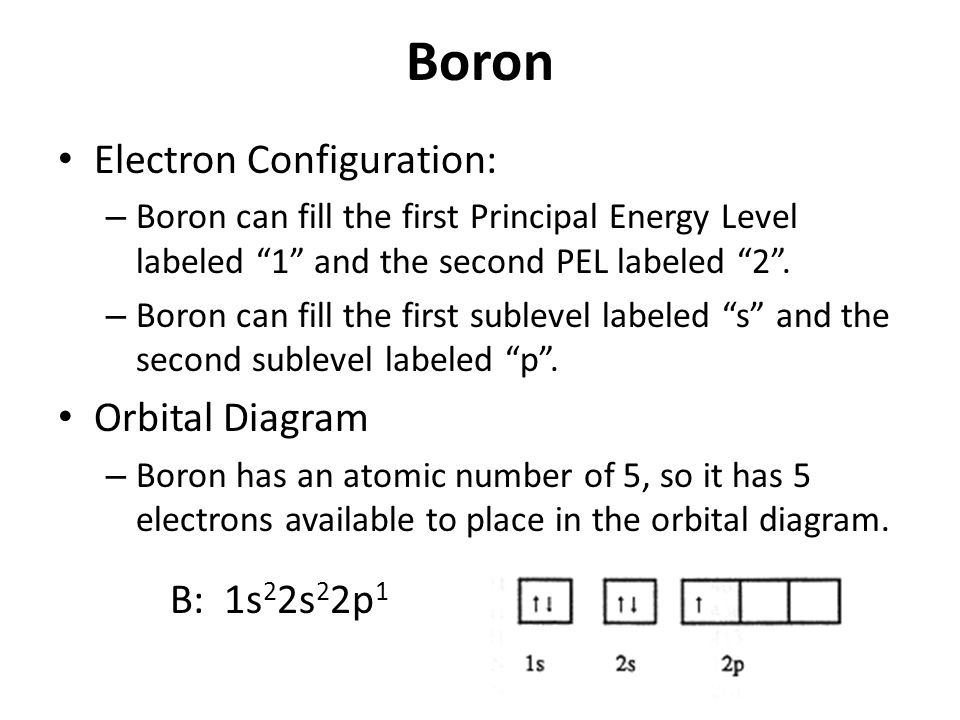
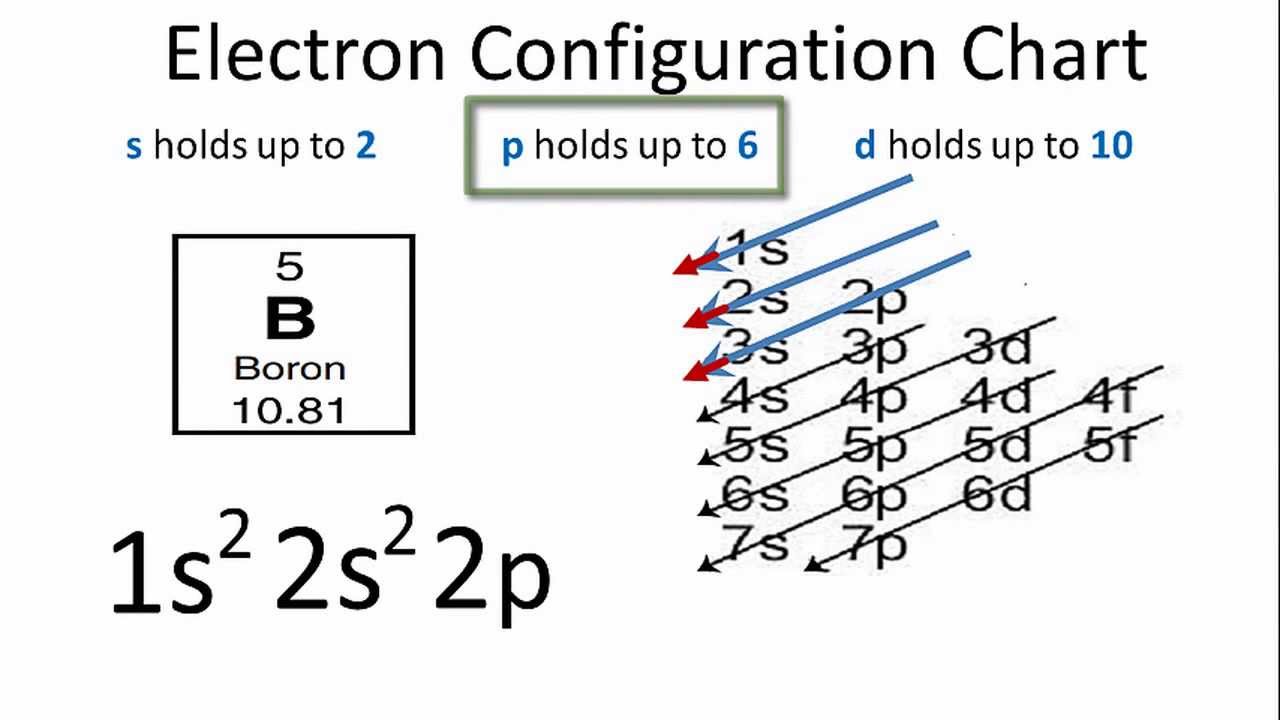
Electron Configuration for Boron (B) - UMD Boron is the fifth element with a total of 5 electrons. In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for B goes in the 2s orbital. The remaining electron will go in the 2p orbital. Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
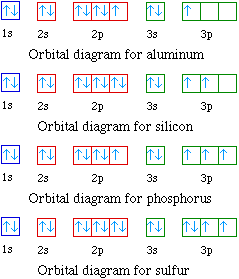
Draw An Orbital Diagram For Boron. Mar 18, 2019 · If you want to learn how to draw orbital filling diagrams, you need to follow The electron configuration of boron is 1s²2s²2p¹, which means that. Boron is the fifth element with a total of 5 electrons. In writing the electron configuration for Boron the first two electrons will go in the 1s orbital.
Complete an orbital diagram for boron.
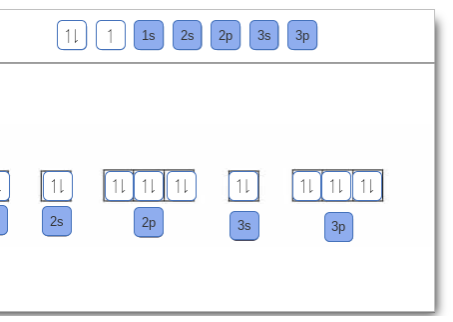
Answered: Part A Complete an orbital diagram for… | bartleby Part A Complete an orbital diagram for boron. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Targets may be left blank, such as for unused orbitals. • View Available Hint(s) Reset Help 11 1s 2s 2p 3s 3p G1 G1 G1| G1 || G1 G1 G1G1 G1 1s 25 2p 35 3p SOLVED:Complete an orbital diagram for boron. Drag the ... Complete an orbital diagram for boron. Drag the appropriate labels to their respective targets: Labels can be used once, more than once; or not at all: View Available Hint(s) Reset Help 2s 2p 35 3p G1 G2 G2 G2 G2 G2 Orbital Filling Diagram For Boron - schematron.org May 11, 2018 · Use this tool to draw the orbital diagram. Draw an orbital diagram for scandium (Sc). From the orbital diagram, we can write the electron configuration in an abbreviated When we reach boron, with Z = 5 and five electrons, we must place the fifth . After filling the first five rows, we still have 80 − 54 = 26 more.Orbital Filling Diagrams.
Complete an orbital diagram for boron.. Arrangements of electrons in the orbitals of an atom is ... The next element is boron with 5 electrons. The orbital diagram for boron as shown has the one electron in the 2p orbital. The electron can be placed in any of the three 2p orbitals. The electron configuration for boron is 1s 2 2s 2 2p 1. (Get Answer) - Complete an orbital diagram for boron ... Complete an orbital diagram for boron Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all Hints Reset Help Complete an orbital diagram for scandium (Sc). Boron(B) electron configuration and orbital diagram Orbital Diagram for Boron (B) Boron (B) excited state electron configuration Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of boron (B) is 1s 2 2s 2 2p 1. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z. Orbital Diagrams [help please :) ] | Yeah Chemistry complete the orbital diagrams for the following atoms. *there little circles, and than you have to fill them in with half arrow things,* 1. Boron
Answered: Complete an orbital diagram for boron.… | bartleby Complete an orbital diagram for boron. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Targets may be left blank, such as for unused orbitals. • View Available Hint(s) Reset Help 1L 1 1s 2s 2p| 3s 3p G1 G1 G1 G1| G1 G1 G1 G1G1 G2 G2 G2 G2 G2 Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of: What are the quantum numbers of the five electrons of Boron? Boron, #"B"#, is located in period 2, group 13 of the periodic table, and has an atomic number equal to #5#.. This means that a neutral boron atom will have a total of #5# electrons surrounding its nucleus.. Now, your tool of choice here will be boron's electron configuration, which looks like this #"B: " 1s^2 2s^2 2p^1# Since you have five electrons, you will need five sets of quantum numbers. Solved Complete an orbital diagram for boron. Drag the ... Complete an orbital diagram for boron. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Complete an orbital diagram; Question: Complete an orbital diagram for boron. Drag the appropriate labels to their respective targets.
Electron Configurations, Orbital Box Notation (M7Q7 ... Thus, the electron configuration and orbital diagram of lithium are: An atom of the alkaline earth metal beryllium, with an atomic number of 4, contains four protons in the nucleus and four electrons surrounding the nucleus. The fourth electron fills the remaining space in the 2s orbital. An atom of boron (atomic number 5) contains five electrons. Chapter 8 Mastering Chemistry Flashcards - Quizlet Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s2 2s2 2p1 is the electron configuration of boron. Use this tool to generate the electron configuration of arsenic (As). Complete An Orbital Diagram For Scandium (sc). An orbital diagram is similar What is the orbital diagram for. For example, write the electron configuration of scandium, Sc: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1. So for scandium the 1 st and 2 nd electron must be in 1s orbital, the 3 rd and 4 th in the 2s, the 5 th through 10 th in the 2p orbitals, etc. 6/14/ Ch 8 4/18 Correct Part B Complete ... Complete An Orbital Diagram For Scandium (sc). Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s22s22p1 is the electron configuration of boron.
what is the electron configuration of boron - Lisbdnet.com In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for B goes in the 2s orbital. The remaining electron will go in the 2p orbital. Therefore the B electron configuration will be 1s22s22p1.
Vanadium(V) electron configuration and orbital diagram Orbital diagram for vanadium (V) Vanadium(V) excited state electron configuration. Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of vanadium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 3 4s 2. The valency of the element is determined by electron configuration in the ...
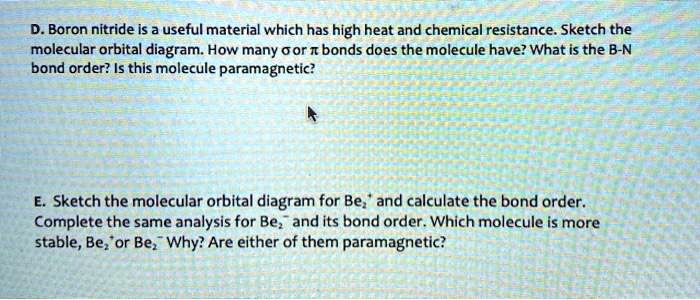
PDF Hybrid Molecular Orbitals - University of Illinois Urbana ... 6. There is one p orbital on boron but there is no adjacent atom with another p orbital. Add it to the molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1.
Part A Complete an orbital diagram for boron Drag the ... Part A Complete an orbital diagram for boron. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Hint 1. How to approach the problem First, determine the number of electrons in an atom of boron ( ). Next, fill the orbitals one electron at a time, from lowest energy to highest energy.
chem 1201 HW Ch. 6 p 2 Flashcards | Quizlet Complete an orbital diagram for boron. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. 1s: 2 arrows 2s: 2 arrows 2p: 1 arrow in the first orbital (leave the rest empty) Complete an orbital diagram for scandium (Sc).
Hund's Rule and Orbital Filling Diagrams | Chemistry for ... Figure 1. The 2p sublevel, for the elements boron (Z = 5), carbon (Z = 6), nitrogen (Z = 7), and oxygen (Z = 8). According to Hund's rule, as electrons are added to a set of orbitals of equal energy, one electron enters each orbital before any orbital receives a second electron.
Solved Complete an orbital diagram for boron. Drag the ... Solved Complete an orbital diagram for boron. Drag the | Chegg.com. Complete an orbital diagram for boron. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets.
What Is The Orbital Diagram For Boron? [Comprehensive Answer] Orbital Filling Diagram For Boron - Hanenhuusholli The electron configuration of boron is 1s²2s²2p¹ which means that there are two electrons in the 1s orbital two electrons in the 2s orbital and one electron in the 2p orbitals. This gives us an orbital filling diagram of. The remaining electron will go in the 2p orbital. Lauren ⭐ Answeregy Expert
What is the orbital diagram for carbon? - MSI What is the orbital diagram for carbon? With beryllium (Z = 4), the 2s sublevel is complete and the 2p sublevel begins with boron (Z = 5). Since there are three 2 p orbitals and each orbital holds two electrons, the 2p sublevel is filled after six elements….Second Period Elements.
Orbital filling diagram of boron? - Answers Because in Boron there is a complete 2s orbital and the increased shielding of the 2s orbital reduces the ionisation energy compared to that seen in Beryllium. What is the orbital diagram for ...
Orbital Filling Diagram For Boron - schematron.org May 11, 2018 · Use this tool to draw the orbital diagram. Draw an orbital diagram for scandium (Sc). From the orbital diagram, we can write the electron configuration in an abbreviated When we reach boron, with Z = 5 and five electrons, we must place the fifth . After filling the first five rows, we still have 80 − 54 = 26 more.Orbital Filling Diagrams.
SOLVED:Complete an orbital diagram for boron. Drag the ... Complete an orbital diagram for boron. Drag the appropriate labels to their respective targets: Labels can be used once, more than once; or not at all: View Available Hint(s) Reset Help 2s 2p 35 3p G1 G2 G2 G2 G2 G2
Answered: Part A Complete an orbital diagram for… | bartleby Part A Complete an orbital diagram for boron. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Targets may be left blank, such as for unused orbitals. • View Available Hint(s) Reset Help 11 1s 2s 2p 3s 3p G1 G1 G1| G1 || G1 G1 G1G1 G1 1s 25 2p 35 3p















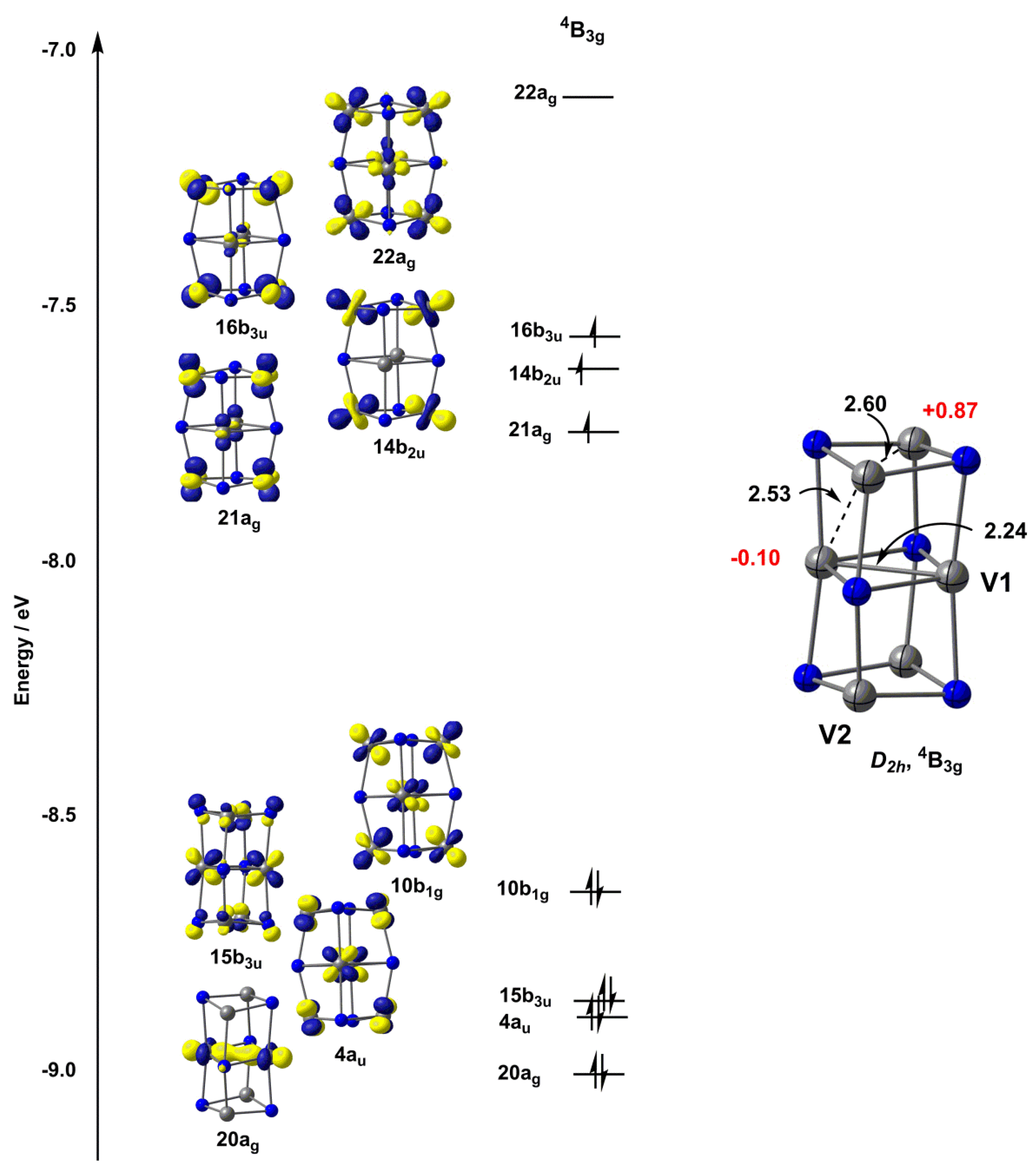



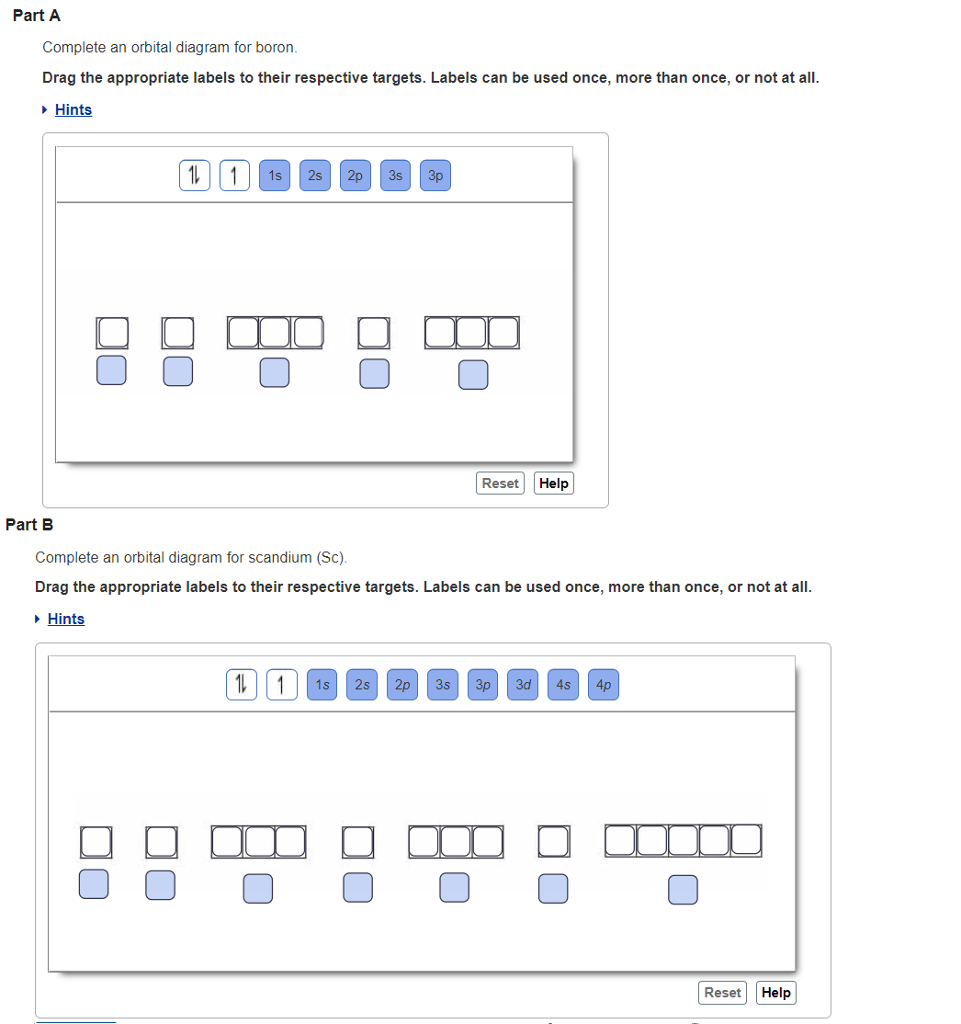





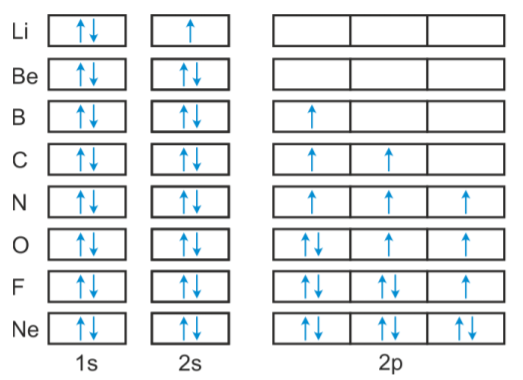
0 Response to "43 complete an orbital diagram for boron."
Post a Comment