43 orbital diagram for lithium
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. Orbital filling diagrams - The Cavalcade o' Chemistry Feb 23, 2016 · The orbital filling diagram of lithium The electron configuration of lithium is 1s²2s¹. This means that there are two electrons in the 1s orbital, and one electron in the higher energy 2s orbital. If you want to make a cool picture, you can do it like this:
Li2- Molecular Orbital Diagram - schematron.org diagram; the MOs go between them. Consider the MO diagram for Li2. This is a bond between two lithium atoms, which have an electron configuration of. The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. The molecule .
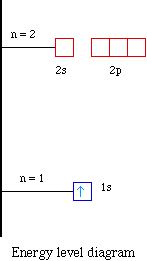
Orbital diagram for lithium
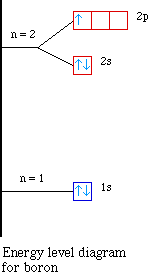
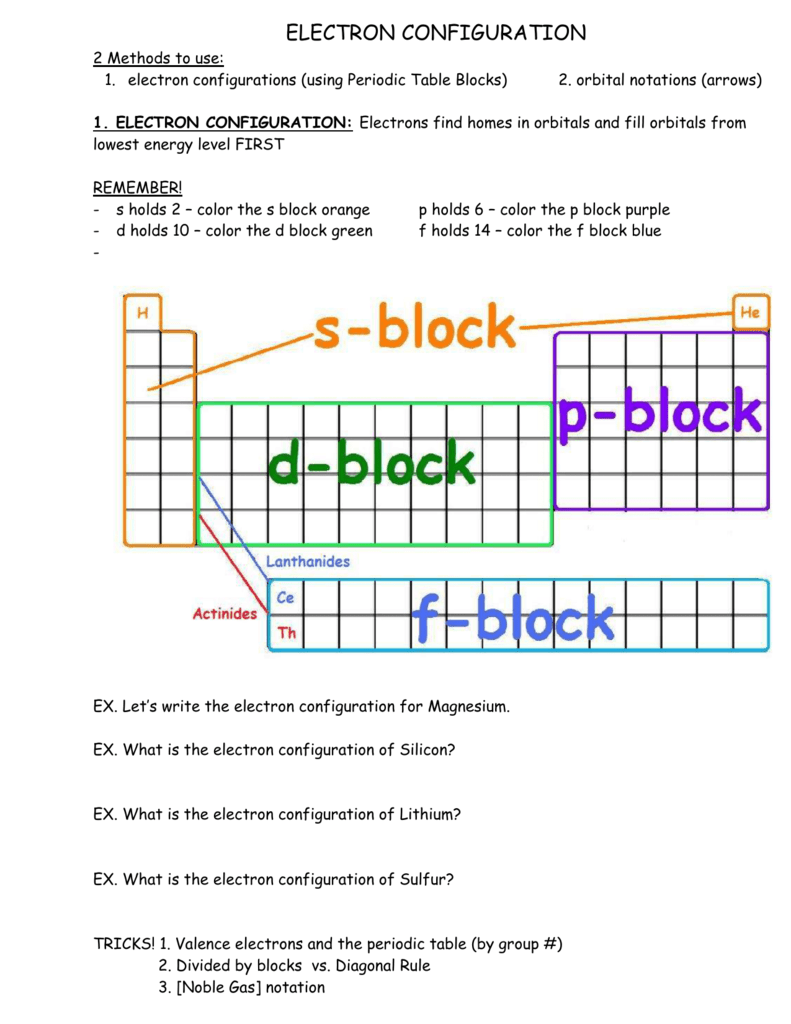
Dublin Schools - Lesson : Orbital diagrams and Electron ... An orbital diagram provides a visual representation of the way in which an atom's electrons are distributed into various orbitals. Each orbital is shown as a single square, and orbitals within the same sub-level are drawn directly next to each other. ... Electron configurations and orbital filling diagrams for lithium through neon are ... Electronic Structure of Atoms (Electron Configurations ... The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom. The next atom is the alkali metal lithium with an atomic number of 3. The first two electrons in lithium fill the 1s orbital and have the same sets of Lithium Bohr Model - How to draw Bohr diagram for Lithium ... According to the Bohr diagram of Lithium, the outer shell is L-shell which contains only 1 valence electron. Properties of Lithium It appears silvery-white in color. It is one of the lightest metal and solid elements. It is highly reactive and flammable. It has a boiling point of 1330 °C and a melting point of 180.50 °C.
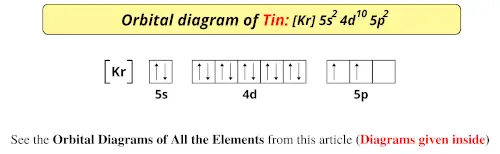
Orbital diagram for lithium. Lithium Orbital diagram, Electron configuration, and ... What is the orbital diagram for Lithium (Li)? The orbital diagram for Lithium is drawn with 2 orbitals. The orbitals are 1s and 2s. The Lithium orbital diagram contains 2 electrons in 1s orbital and the remaining one electron in 2s orbital. The orbital diagram for a ground-state electron configuration of the Lithium atom is as follow – Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ... Draw and explain the M.O. diagram of lithium molecule ... Draw the M.O diagram for oxygen molecule and calculate its bond order and show that O2 is paramagnetic. asked Dec 17, 2020 in Chemical Bonding by Panna01 ( 47.2k points) chemical bonding Molecular Orbital Diagram of Lithium Molecule - Nature of ... Molecular Orbital Diagram of Lithium Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET....
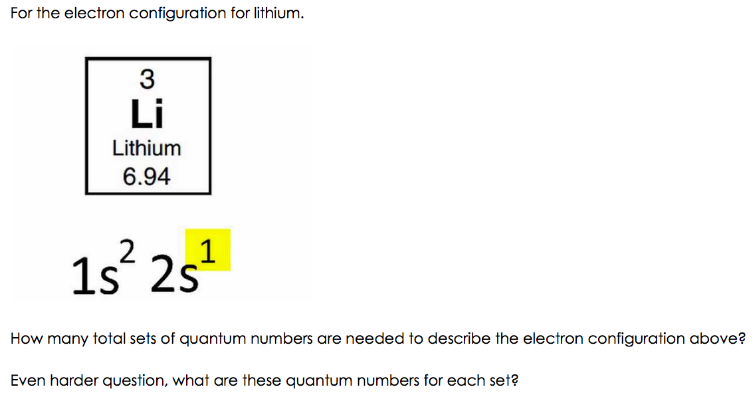
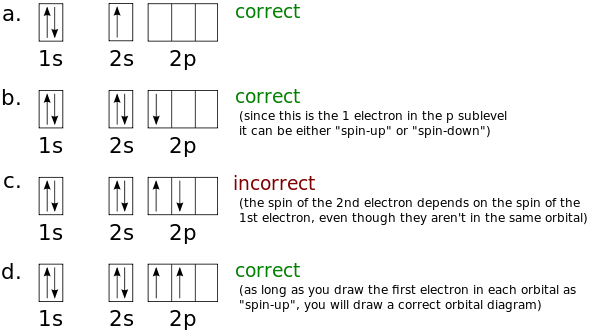
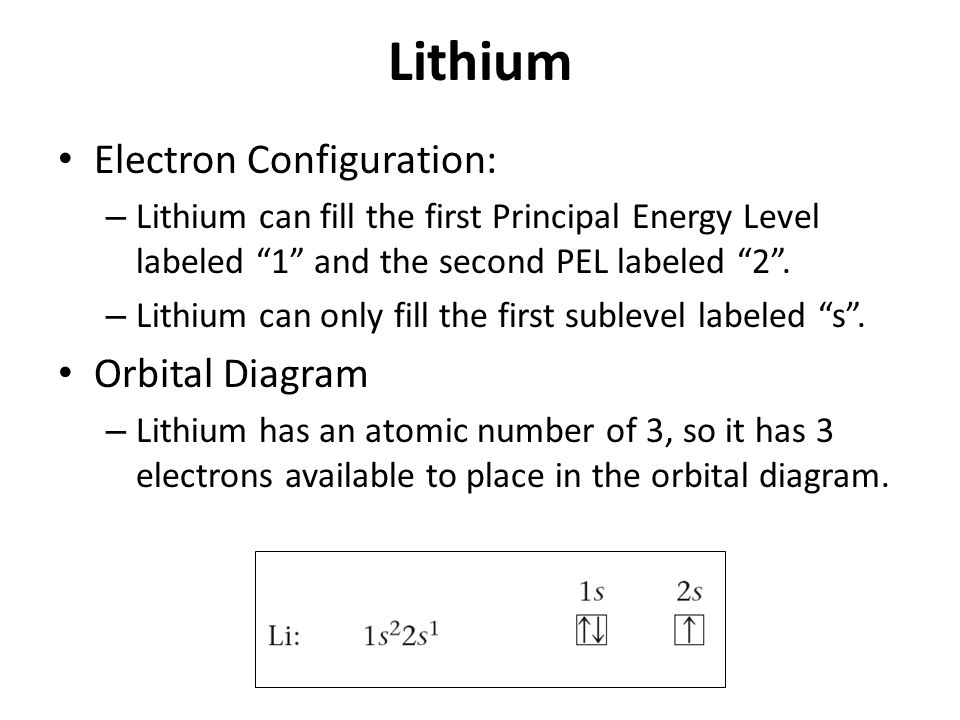
Lithium(Li) electron configuration and orbital diagram Lithium (Li) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. Arrangements of electrons in the orbitals of an atom is ... So the orbital diagram for lithium is shown below. The electron configuration for lithium is 1s 2 2s 1 . The energy level diagram, on the left shows the relative energy of the 2s and 2p orbitals based on the ability of the sublevels to penetrate to the nucleus. The next element is beryllium which has four electrons. M7Q7: Electron Configurations, Orbital Box Notation - Chem ... The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2s orbital (Figure 3 or Figure 4). Thus, the electron configuration and orbital diagram of lithium are: What is the orbital diagram of lithium? - Answers Dec 01, 2010 · ok so you'll notice that lithium is on the second row of the periodic table, this means that its the next orbital hydrogen = 1s1 orbital helium = 2s1 orbital lithium = 2s1, 1s2 orbital removing the...
Iridium(Ir) electron configuration and orbital diagram Iridium (Ir) electron configuration and orbital diagram. Iridium is the 77th element in the periodic table and its symbol is 'Ir'. Iridium is a classified transition metal element. The total number of electrons in iridium is seventy-seven. These electrons are arranged according to specific rules of different orbits. What Is The Molecular Orbital Configuration Of Lithium ... A molecular orbital can hold two electrons, so both electrons in the H 2 molecule are in the σ 1s bonding orbital; the electron configuration is (σ1s)2. We represent this configuration by a molecular orbital energy diagram (Figure 8.4. What is the electronic configuration of F2? Answer: F2 the following: (sigma 2s)^2. (sigma 2s*)^2. Electron Configurations, Orbital Box Notation (M7Q7 ... Thus, the electron configuration and orbital diagram of lithium are: An atom of the alkaline earth metal beryllium, with an atomic number of 4, contains four protons in the nucleus and four electrons surrounding the nucleus. The fourth electron fills the remaining space in the 2 s orbital. An atom of boron (atomic number 5) contains five electrons. Solved Fill in the orbital energy diagram for the lithium ... Chemistry. Chemistry questions and answers. Fill in the orbital energy diagram for the lithium atom. 2p 1s.
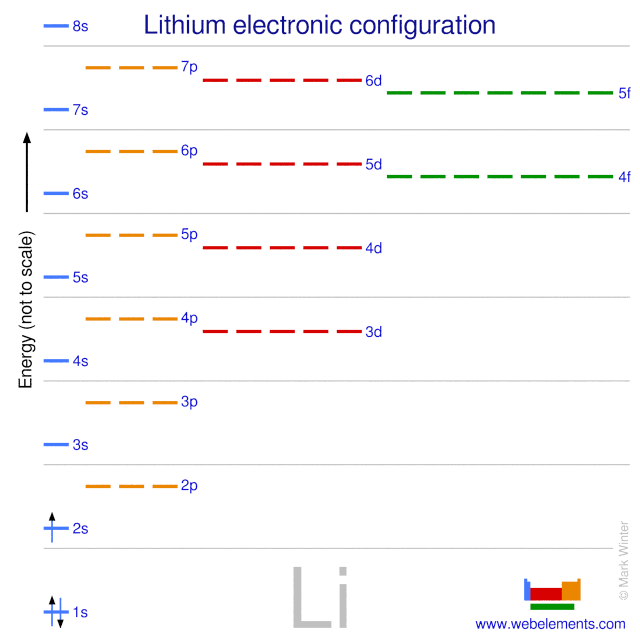
Hydrogen-Like Atoms:Lithium - Georgia State University The illustration above uses the hydrogen wavefunctions, which are not exactly correct for lithium but can be used to obtain a qualitative understanding of the dependence of the electron energies on the orbital quantum number. If there were no shielding of the 2s electron, it would be exposed to the entire nuclear charge and have energy -30.6 eV.
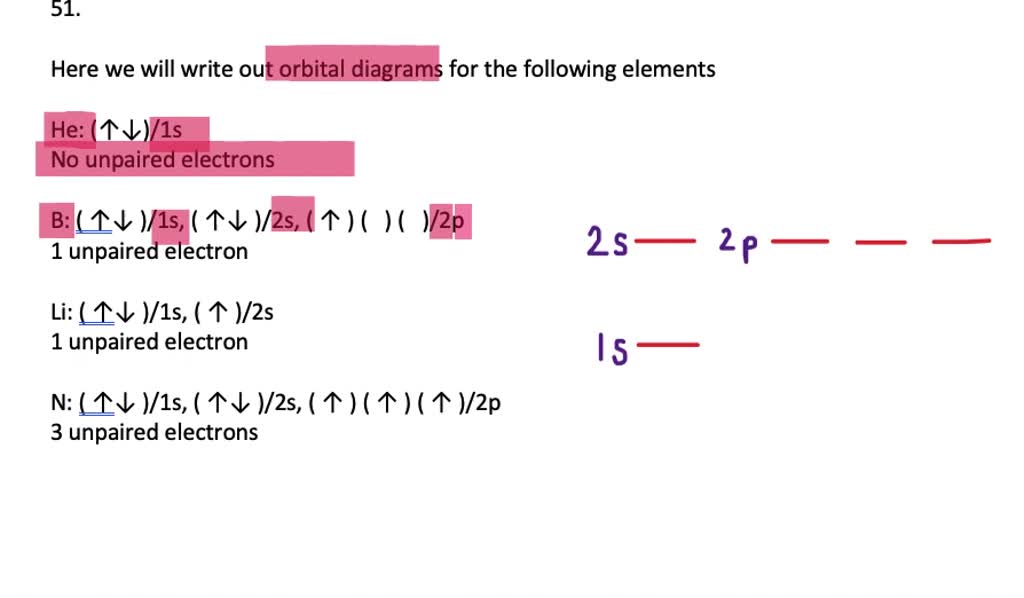
Electron Configurations | Chemistry for Non-Majors Sample Problem: Orbital Filling Diagrams and Electron Configurations. Draw the orbital filling diagram for carbon and write its electron configuration. Step 1: List the known quantities and plan the problem. Known . atomic number of carbon, Z = 6; Use the order of fill diagram to draw an orbital filling diagram with a total of six electrons.
SOLVED:Fill in the orbital energy diagram for the lithium ... Its orbital diagram will keep the noble gas core of zen on, and then we have four F. So that's seven possible orbital's 4567 And then we have six s, which has one orbital, so for four F will have six electrons.
How to Write the Orbital Diagram for Lithium (Li) - YouTube To write the orbital diagram for the Lithium atom (Li) first we need to write the electron configuration for just Li. To do that we need to find the number ...
Copper(Cu) electron configuration and orbital diagram Therefore, an electron of 4s orbital completes a full-filled 3d orbital by jumping into a 3d orbital. So, the copper(Cu) electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. How to write the orbital diagram for copper(Cu)? To create an orbital diagram of an atom, you first need to know Hund's principle and Pauli's exclusion ...
Dublin Schools - Lesson : Orbital diagrams and Electron ... The orbital filling diagrams for hydrogen, helium, and lithium are shown below. According to the Aufbau principle, sub-levels and orbitals are filled with electrons in order of increasing energy. Since the s sub-level consists of just one orbital, the second electron simply pairs up with the first electron, as in helium.
Answered: Fill in the orbital energy diagram for… | bartleby Science Chemistry Q&A Library Fill in the orbital energy diagram for the lithium ion. 2p- E 2s- 1s-Fill in the orbital energy diagram for the lithium ion. 2p- E 2s- 1s-Question. fullscreen Expand. Transcribed Image Text. Fill in the orbital energy diagram for the lithium ion. 2p E 2s 1s check_circle
Electron Configuration for Lithium (Li) Lithium is the third element with a total of 3 electrons. In writing the electron configuration for lithium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the remaining electron for Li goes in the 2s orbital. Therefore the Li electron configuration will be 1s 2 2s 1.
Calcium Orbital Filling Diagram - schematron.org The orbital filling diagram of lithium. The electron configuration of lithium is 1s²2s¹. This means that there are two electrons in the 1s orbital, and one electron in the higher energy 2s orbital. When we write the configuration we'll put all 20 electrons in orbitals around the nucleus of the Calcium atom.
Hund's Rule and Orbital Filling Diagrams | Chemistry for ... Orbital filling diagrams for hydrogen, helium, and lithium. According to the Aufbau process, sublevels and orbitals are filled with electrons in order of increasing energy. Since the s sublevel consists of just one orbital, the second electron simply pairs up with the first electron as in helium.
Molecular Orbitals - Chem1 The lithium 1sorbital is the lowest-energy orbital on the diagram. Because this orbital is so small and retains its electrons so tightly, it does not contribute to bonding; we need consider only the 2sorbital of lithium which combines with the 1sorbital of hydrogen to form the usual pair of sigma bonding and antibonding orbitals.
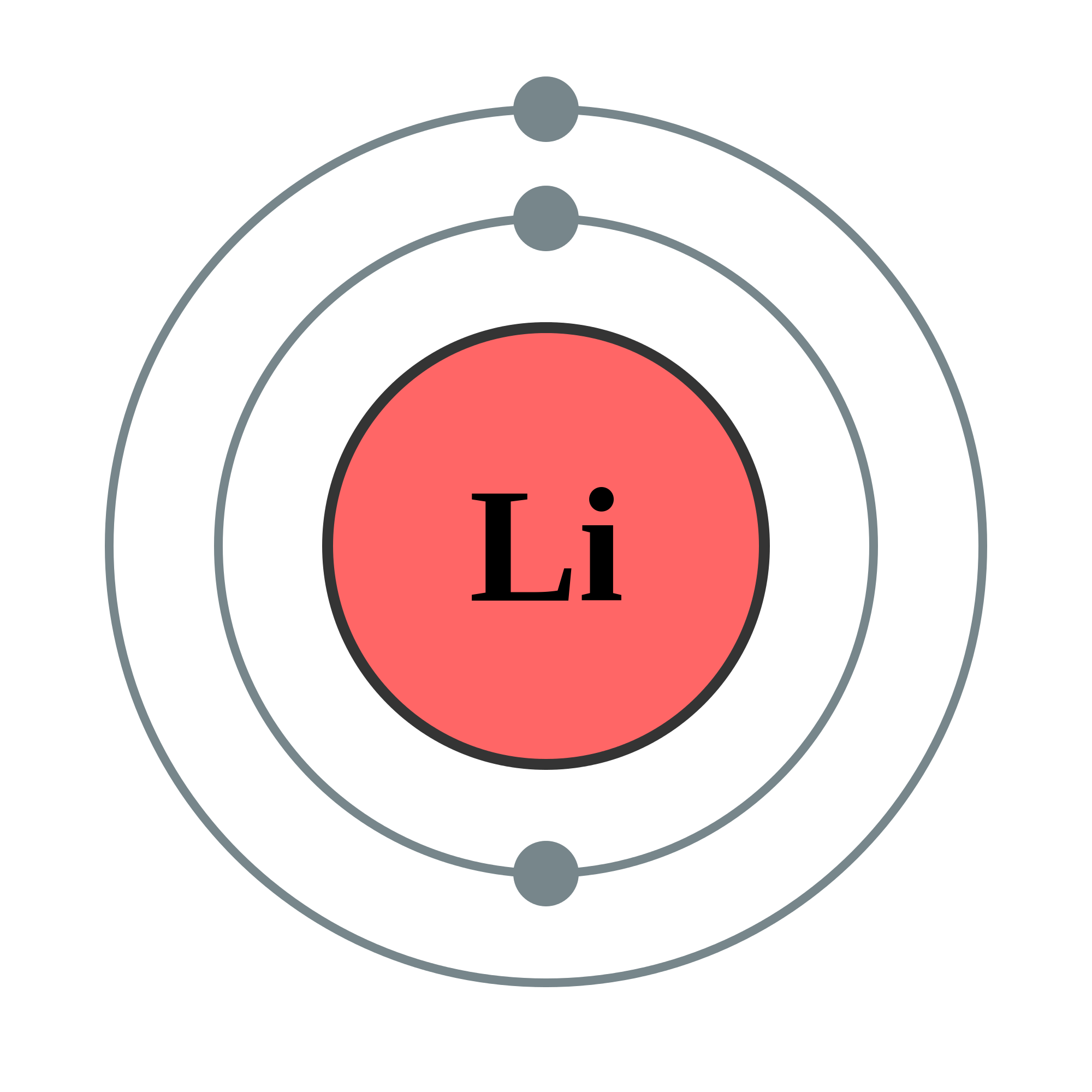
Lithium Bohr Model - How to draw Bohr diagram for Lithium ... According to the Bohr diagram of Lithium, the outer shell is L-shell which contains only 1 valence electron. Properties of Lithium It appears silvery-white in color. It is one of the lightest metal and solid elements. It is highly reactive and flammable. It has a boiling point of 1330 °C and a melting point of 180.50 °C.
Electronic Structure of Atoms (Electron Configurations ... The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom. The next atom is the alkali metal lithium with an atomic number of 3. The first two electrons in lithium fill the 1s orbital and have the same sets of
Dublin Schools - Lesson : Orbital diagrams and Electron ... An orbital diagram provides a visual representation of the way in which an atom's electrons are distributed into various orbitals. Each orbital is shown as a single square, and orbitals within the same sub-level are drawn directly next to each other. ... Electron configurations and orbital filling diagrams for lithium through neon are ...

















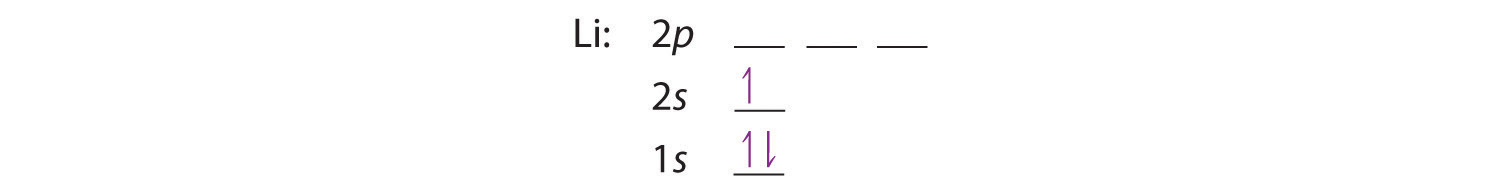


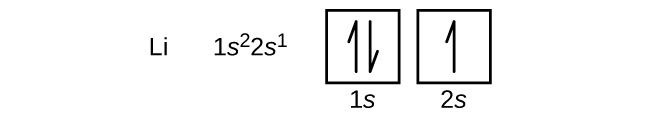

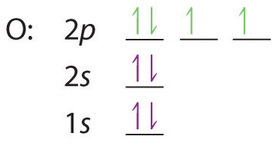





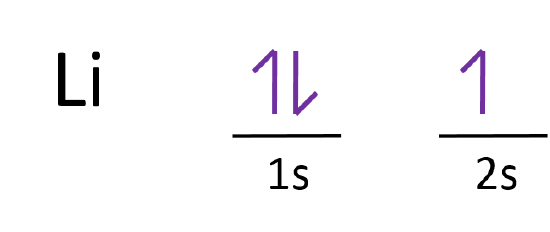
0 Response to "43 orbital diagram for lithium"
Post a Comment