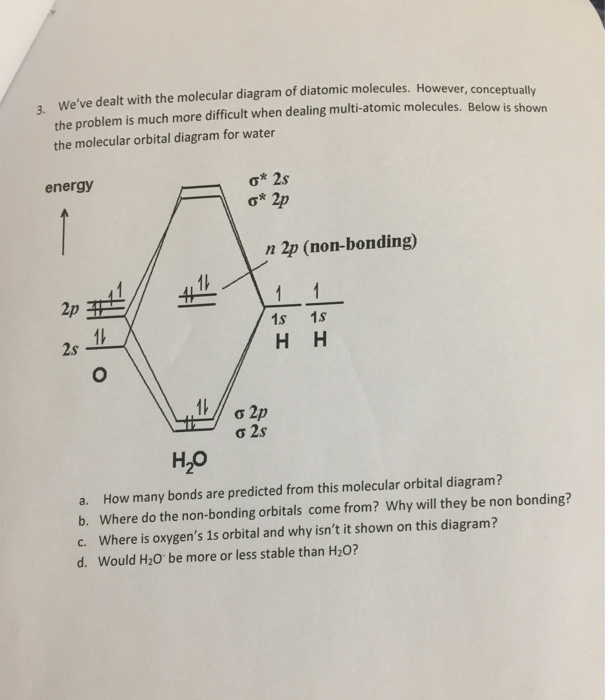
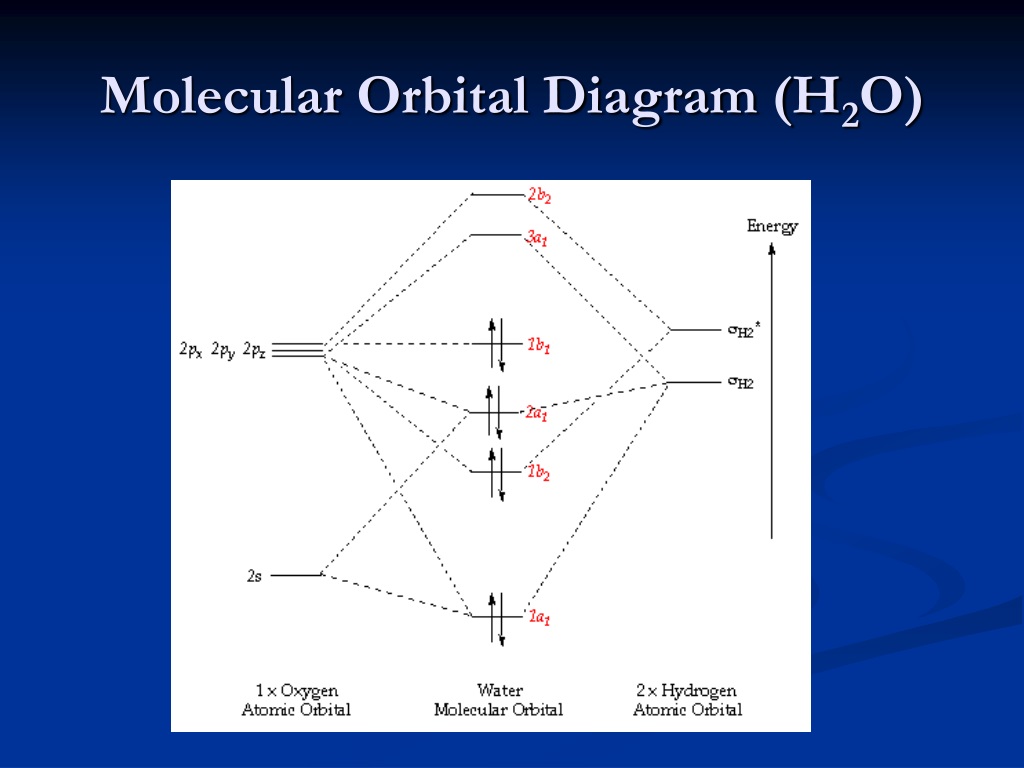
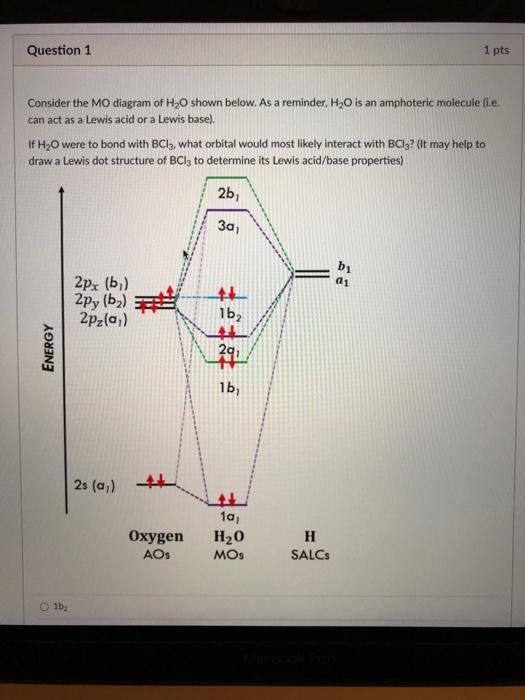
45 molecular orbital diagram for h2o
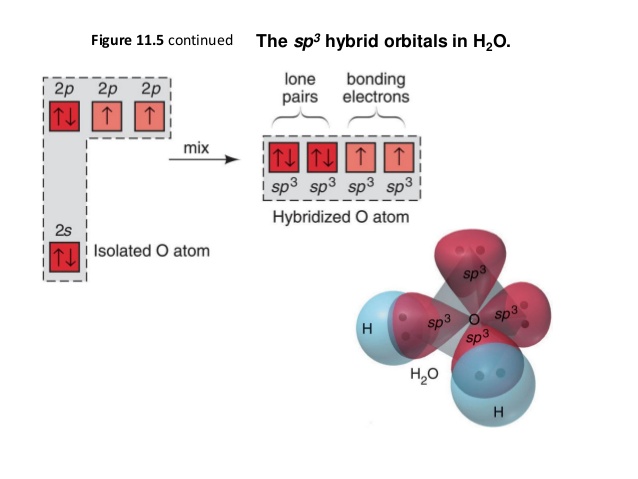
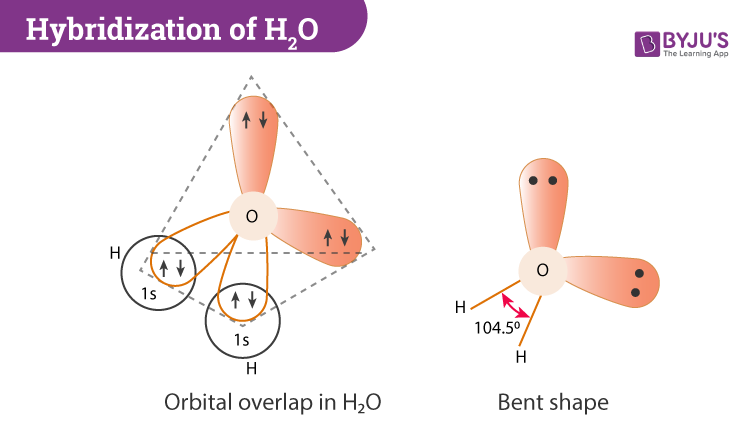
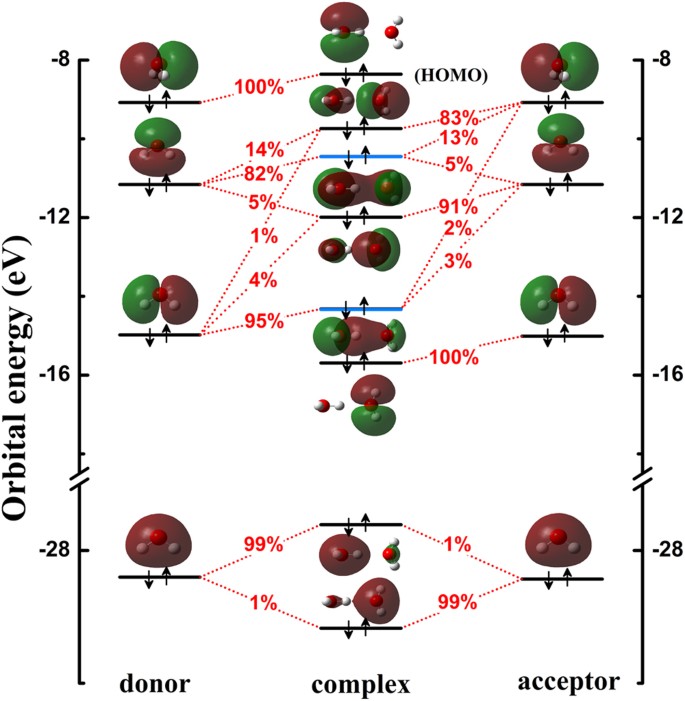
PDF Figure 9.32: The molecular orbital energy-level diagram for • Because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable. Figure 9.26: (a) The molecular orbital energy-level diagram for the H2 molecule. (b) The shapes of the molecular orbitals are obtained by squaring the wave functions for MO1 and... Molecular Structure & Bonding For molecules of water and ammonia, however, the non-bonding electrons must be included in the calculation. In each case there are four regions of electron density associated with the valence shell so that a tetrahedral bond angle is expected. The measured bond angles of these compounds (H2O...
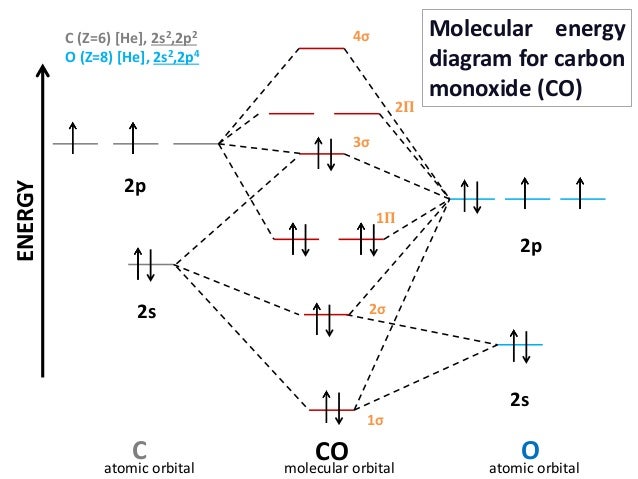
Fig. No. 4 Molecular orbital energy level diagram for N2 molecule. The molecular orbital electronic configuration of Homonuclear Diatomic Molecule H2 is: Therefore, there is a covalent bond between the two hydrogen Therefore, this ion consists of only one electron. The molecular orbital diagram is given in figure below. Fig. No. 2 Molecular Orbital Energy Level...

Molecular orbital diagram for h2o
H3O+ Molecular Orbital (MO) Diagram - Techiescientist A molecular orbital diagram of any molecule gives us an idea about the mixing of orbitals in the molecule. Talking about the overlap diagram of H3O+, it is almost similar to H2O but with one electron less and one hydrogen more. Given below is the image of the molecular orbital diagram of... File:H2O-MO-Diagram.svg - Wikimedia Commons File:H2O-MO-Diagram.svg. From Wikimedia Commons, the free media repository. Jump to navigation Jump to search. Added orbital diagrams for molecular orbitals. molecular orbital energy-level diagram | Britannica The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H2 molecule is shown in Figure 13. On either side of the central ladder are shown the energies of the 1s orbitals of atoms A and B
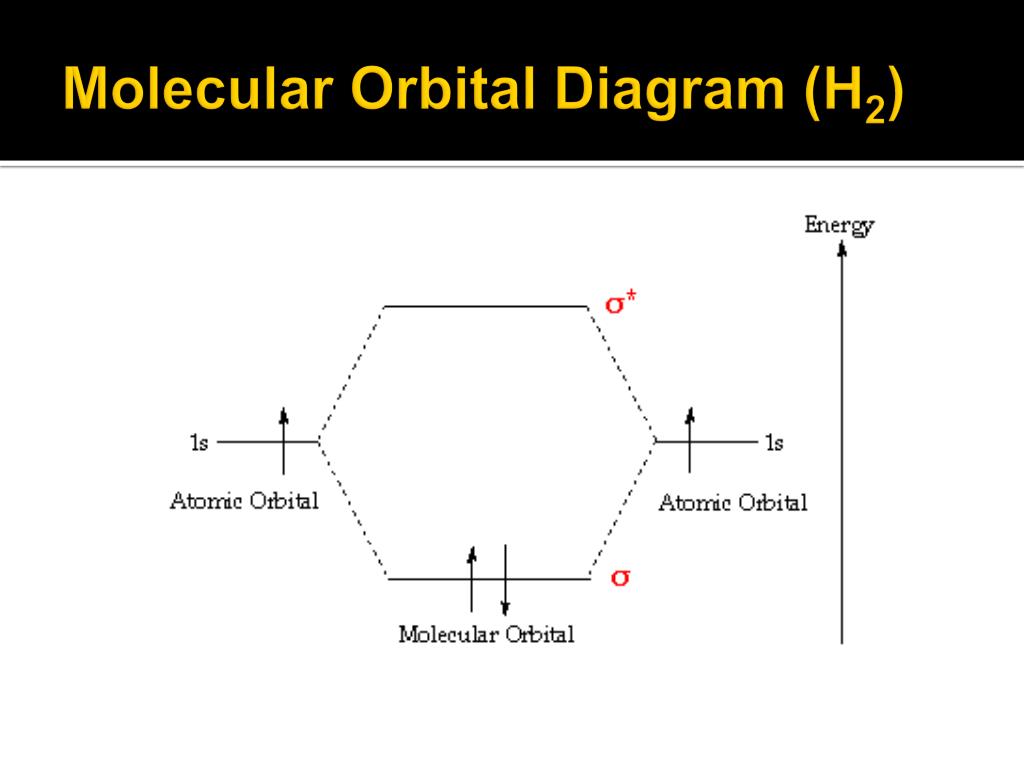
Molecular orbital diagram for h2o. Molecular Orbital Theory | Boundless Chemistry In molecular orbital theory, bond order is also defined as the difference, divided by two, between the number of bonding and antibonding electrons; this often, but not always, yields the same result. This MO diagram depicts the molecule H2, with the contributing AOs on the outside sandwiching the MO. Figure 2. Molecular orbital energy diagrams for (a) H 2 showing both... The molecular orbital (MO) theory is a way of looking at the structure of a molecule by using molecular orbitals that belong to the molecule as a The energy diagrams are shown in Figure 2. Each hydrogen atom has one 1 s electron. In the H 2 molecule the two hydrogen electrons go into... 5.4 Molecular Orbital Theory - Chemistry: Atoms First 2e | OpenStax The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. Figure 5.35 The molecular orbital energy diagram predicts that H2 will be a stable molecule with lower energy than the separated atoms. Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
Molecular Orbital Theory - ppt video online download 1 Molecular Orbital Theory The goal of molecular orbital theory is to describe molecules in a similar way to how we describe atoms, that is, in terms of orbitals, orbital diagrams, and electron configurations. This is an illustration of molecular orbital diagram of H2. PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams Molecular orbital calculations indicate, however, that for O2, F2, and hypothetical Ne2 molecules, the 2p orbital is lower in energy than the 2p orbitals The solid lines represent the relative energies of the indicated atomic and molecular orbitals. (a) The diagram for H2, He2, Li2, Be2, B2, C2, and N2... Molecular Orbital Theory This molecular orbital model can be used to explain why He2 molecules don't exist. Combining a pair of helium atoms with 1s2 electron configurations would The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the... Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
Molecular Orbital (MO) Diagram of H2 - YouTube Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me... Molecular Orbital Theory - Chemistry | Socratic Molecular orbital theory is a method for determining molecular structure. Thus, Molecular Orbital theory explains resonance delocalization automatically as the natural state of the molecule. How do you draw an atomic level diagram for 2S+3O2=2SO3 ? PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... - MO diagrams for Inorganic complexes. • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital Theory. PDF Procedure for Constructing Molecular Orbital Diagrams Based on... Last time you learned how to construct molecule orbital diagrams for simple molecules based on the symmetry of the atomic orbitals. We could use the symmetry-based method to construct molecular orbital diagrams for larger molecules as well, but this can get complicated for larger structures.
Molecular Orbitals: Molecular Orbital Theory | SparkNotes In the middle of the diagram, the molecular orbitals of the molecule of interest are written. Because hydrogen has one electron pair in its bonding orbital and none in its antibonding orbital, molecular orbital theory predicts that H 2 has a bond order of one--the same result that is derived from Lewis...
Why is the molecular orbital diagram for O₂ different from N₂? - Quora If we compare such diagrams for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂, and F₂), the resulting pattern looks like this Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons.
PDF Slide 1 Molecular orbital theory for diatomic molecules. Thus we can draw ENERGY LEVEL DIAGRAM for m.o.'s of H2 Molecular Orbital Energy Level Diagram for a Heteronuclear Diatomic.
Molecular Orbital diagram of NO(nitric oxide) molecule Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams, Chemistry Viva, Chemistry Molecular Orbital diagram of O2- ion : This is superoxide ion.
MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
Asked for: molecular orbital energy-level diagram, valence electron... Thus molecular orbital theory correctly predicts that H2 is a stable molecule. Because bonds form when electrons are concentrated in the space between nuclei, this Draw the molecular orbital energy-level diagram for the system. Determine the total number of valence electrons in the He22+ ion.
Tutorial on Chemical Bonding, Part 8 of 10 (Molecular orbitals) The diagram shows how the molecular orbitals in lithium hydride can be related to the atomic orbitals of the parent atoms. Notice that the relative energies of the 2p-derived σ and π bonding molecular orbitals are reversed in O2 and F2. This is attributed to interactions between the 2s orbital each atom...
Energy level diagram for Molecular orbitals - Chemical Bonding and... 3) If Nb = Na ,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding Greater value of bond order for H2 molecule than H2+ ion shows that two H2 molecule is more stable than H2+.
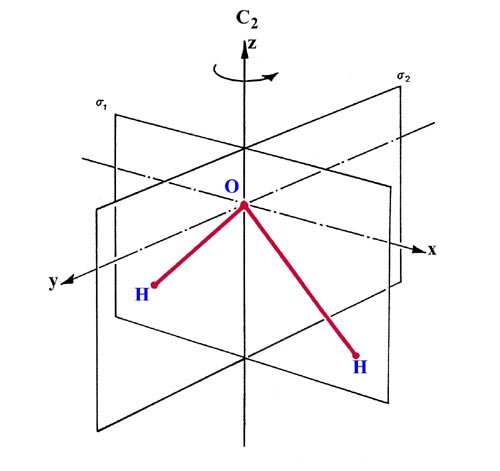
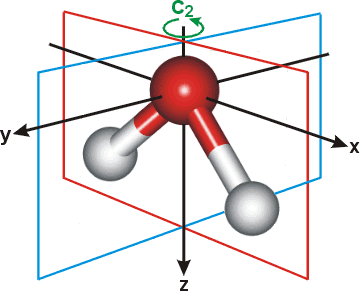
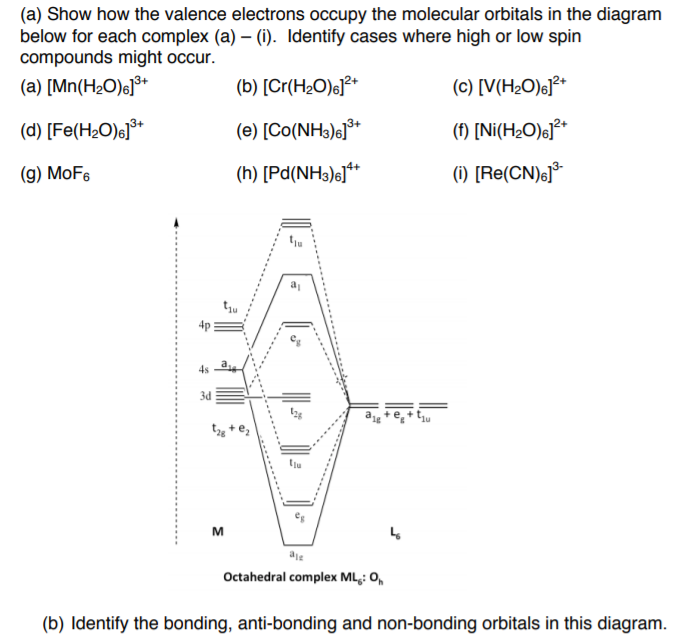
PDF Molecular Orbitals Molecular Orbital Diagrams. 1. Electrons preferentially occupy molecular orbitals that are lower in energy. 2. Molecular orbitals may be empty, or contain one or two electrons. When a central atom is bonded to several atoms of the same element (H2O, BF3, or PtCl42-], group theory can be used to...
8.4 Molecular Orbital Theory - Chemistry Figure 9. The molecular orbital energy diagram predicts that H2 will be a stable molecule with lower energy than the separated atoms. Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule.
Molecular Orbital Diagrams simplified | by Megan A. Lim | Medium Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory. Valence Bond Theory proposes that electrons are localized between two atoms.
MO's for 2nd row diatomic molecules Molecular Orbital theory (MO) is the most important quantum mechanical theory for describing bonding in molecules. However, we can easily imagine this for a diatomic system such as H2. For they hydrogen molecule there is only one thing we can change, the distance between the two nuclei.
molecular orbital energy-level diagram | Britannica The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H2 molecule is shown in Figure 13. On either side of the central ladder are shown the energies of the 1s orbitals of atoms A and B
File:H2O-MO-Diagram.svg - Wikimedia Commons File:H2O-MO-Diagram.svg. From Wikimedia Commons, the free media repository. Jump to navigation Jump to search. Added orbital diagrams for molecular orbitals.
H3O+ Molecular Orbital (MO) Diagram - Techiescientist A molecular orbital diagram of any molecule gives us an idea about the mixing of orbitals in the molecule. Talking about the overlap diagram of H3O+, it is almost similar to H2O but with one electron less and one hydrogen more. Given below is the image of the molecular orbital diagram of...

























0 Response to "45 molecular orbital diagram for h2o"
Post a Comment