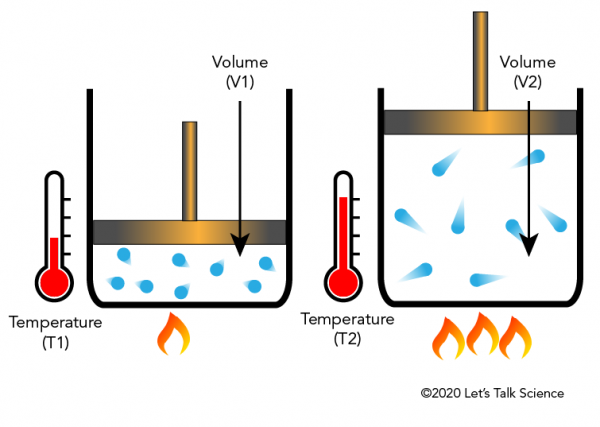
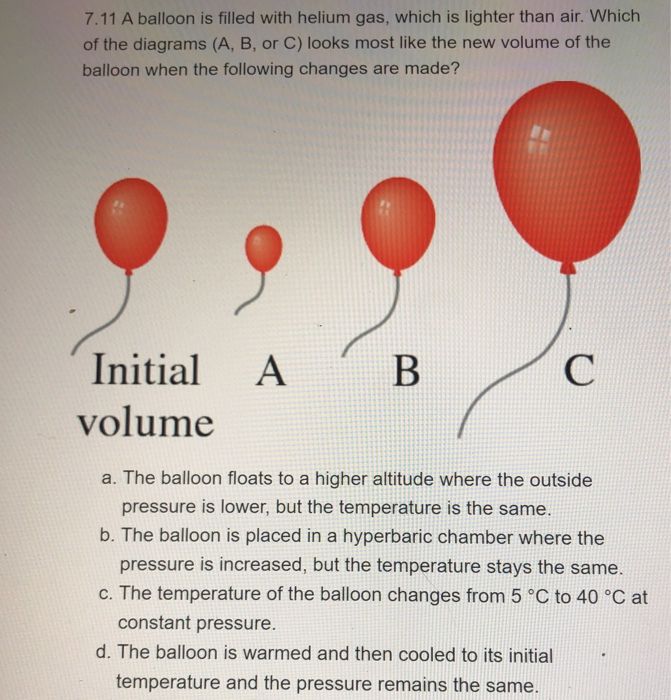
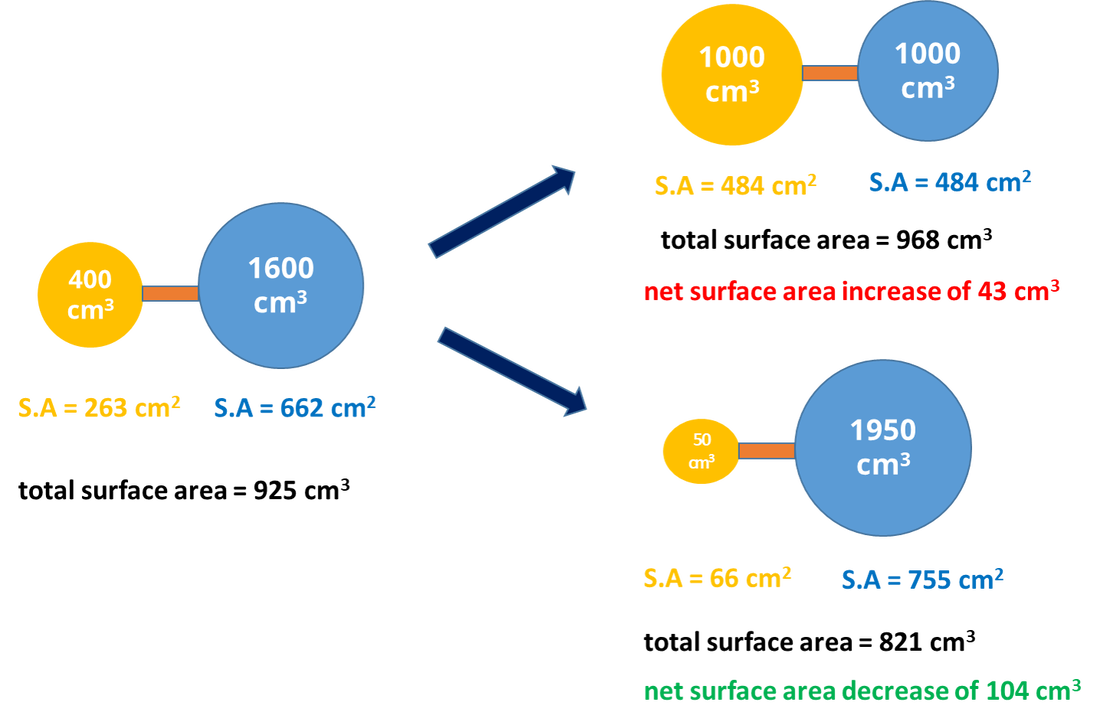
45 one way to increase the volume of the gas in the balloon in the diagram above is to -
9.2 Relating Pressure, Volume, Amount, and Temperature: The ... At STP, an ideal gas has a volume of about 22.4 L—this is referred to as the standard molar volume . Figure 10. Since the number of moles in a given volume of gas varies with pressure and temperature changes, chemists use standard temperature and pressure (273.15 K and 1 atm or 101.325 kPa) to report properties of gases. 5 Dimension 3: Disciplinary Core Ideas - Physical Sciences ... A stable system is one in which the internal and external forces are such that any small change results in forces that return the system to its prior state (e.g., a weight hanging from a string). A system can be static but unstable, with any small change leading to forces that tend to increase that change (e.g., a ball at the top of a hill). A ...
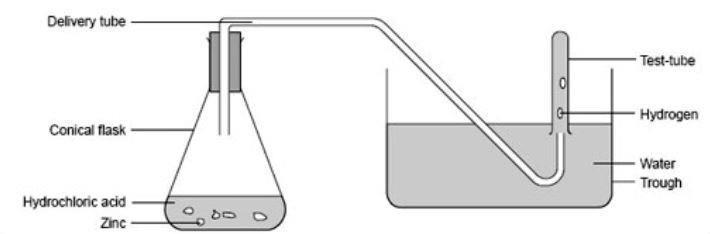
The Kinetic-Molecular Theory - Chemistry What is the lifting power of such a balloon? If the weight of the balloon and its rigging is 500 pounds, what is its capacity for carrying passengers and cargo? (g) A balloon carries 40.0 gallons of liquid propane (density 0.5005 g/L). What volume of CO 2 and H 2 O gas is produced by the combustion of this propane?
One way to increase the volume of the gas in the balloon in the diagram above is to -
Unit 1 Review: Properties of Matter Quiz - Quizizz Q. An experiment was performed to determine the specific heat of some common substances. Using the information in the data table above, choose the most reasonable conclusion. answer choices. Metals require more energy than nonmetals to raise the temperature 1 k. Lead requires the most energy to raise its temperature 1k. One way to increase to volume of the gas in the balloon ... If the volume of gas in the balloon remains constant, then an increase in temperature would result in an increased gas pressure in a balloon.That result can be achieved in three ways:1). Pump more... PDF Chem. 100 Experiment Name: Chemistry 100 Laboratory ... The temperature and volume of a gas are directly proportional. If the temperature of a gas is doubled then the volume is doubled if the pressure remains the same. V 1 = V 2 T 1 T 2 Pressure Standard pressure is 1 atmosphere (atm) or 760 mm Hg Temperature When doing gas calculations the Celsius temperature must be changed to Kelvin.
One way to increase the volume of the gas in the balloon in the diagram above is to -. 6.4 Density, mass and volume | Particle model of matter ... The amount of space that an object occupies is called its volume. Volume is measured in litres and is calculated by multiplying the length, width and height of an object. A litre is the space inside a cube that is 10 cm wide, 10 cm long and 10 cm deep. When calculating volume, 1 cm x 1 cm x 1 cm = 1 cm 3. Physics-SchoolUK.com - Particle Model of Matter KS4. In the section above we explained briefly why a gas produces a pressure within a container and why the pressure increases/decreases as the volume decreases/increases. ... A balloon has a volume of 0.014 m 3 and the air inside is at a pressure of ... one way to increase the pressure of a gas is to fix the volume of its container but increase its ... Physical Science 1/25 Flashcards - Quizlet -ping pong balls in an upside down funnel that doesn't fall boyle's law -if you decrease the volume of a container of gas and hold the temp constant, the pressure from the gas will increase -P1V1=P2V2 -gas -when you squeeze a balloon and it pops Charles' Law -the volume of a gas increases with increasing temp -V1/T1=V2/T2 -gases What happen to the balloon when it ... - UCSB Science Line Does its size increase or decrease? Question Date: 2008-09-09: Answer 1: Well lets see. Let us say you inflate the balloon at the surface where the pressure is 1 bar (10 5 Pa). Now in the balloon, the pressure of the gas is slightly greater than one bar because the strength of the elastic balloon.
JLab Chemistry Questions Flashcards | Quizlet 7. One example of an ionic compound is -. MgCl2. Calcium carbonate was placed in a flask on a balance, and dilute hydrochloric acid was added. Carbon dioxide that was produced escaped from the flask. The total mass of the flask and its contents was recorded every 10 seconds. The diagram above shows a plot of the results. The diagram represents one way an enzyme can be inhibited One way to increase the volume of the gas in the balloon in the diagram above is to - In the diagram, which one represents a hypertonic solution The diagram represents one of mendel's laws or principles of inheritance. Energy can be transformed from one form to another. the diagram shows one such process. Chemistry: Unit 1.18 Test Sem 2 Flashcards - Quizlet a point at which if you go above, you can no longer distinguish between a liquid and a gas The average speeds of gas molecules in cylinders A, B, C, and D are 0.0002 m/s, 0.03 m/s, 0.5 m/s, and 0.8 m/s, respectively. Circular Motion and Gravitation Review - Answers #1 An increase in M results in a proportional increase in g. H is false; g is approximately 10 m/s/s on earth's surface. Doubling the mass of the earth would increase g to approximately 20 m/s/s. Then doubling the distance from the surface of the earth to its center would decrease g by a factor of 4. The new acceleration of gravity value would be ...
Achiever Student: Easy way to better grades. We write custom essay samples to help international students succeed with their studies Order your paper. Grades. We will help you score well in that assignment! 96%. our average grade score. We always make sure that writers follow all your instructions precisely. You can choose your academic level: high school, college/university, … Gas - Wikipedia Gas is one of the four fundamental states of matter (the others ... The volume of the balloon in the video shrinks when the trapped gas particles slow down with the addition of extremely cold nitrogen. The temperature of any physical system is related to the motions of the particles (molecules and atoms) which make up the [gas] system. In statistical mechanics, temperature … Gas Laws - Department of Chemistry & Biochemistry Think of it this way, if you increase the volume of a gas and must keep the pressure constant the only way to achieve this is for the temperature of the gas to increase as well. Calculations using Charles' Law involve the change in either temperature (T 2 ) or volume (V 2 ) from a known starting amount of each (V 1 and T 1 ): 3 Ways To Increase the Pressure of a Gas - ThoughtCo Three Ways to Increase the Pressure of a Gas . Increase the amount of gas. This is represented by the "n" in the equation. Adding more molecules of a gas increases the number of collisions between the molecules and the walls of the container. This raises pressure. Increase the temperature of the gas. This is represented by "T" in the equation.
Final E109 Flashcards | Quizlet 1) Sequeezing Balloon A (yellow) results in no volume of Balloon A while the volume of Balloon B decreases. 2) squeezing the Balloon B (blue) causes an increase in the volume of Balloon A while the volume of Balloon B decreases. Choose the diagram that beat represents the system you were given.
Chapter 5 AP Chem Finals Review Flashcards - Quizlet 1) Represented above are five identical balloons, each filled to the same volume at 25 C and 1.0 atm pressure with the pure gas indicated. a) Which balloon contains the greatest mass of gas? Explain b) Compare the average kinetic energies of the gas molecules in the balloons.
PDF 1994 B Represented above are five identical balloons, each filled to the same volume at 25 C and 1.0 atmosphere pressure with the pure gases indicated. (a) Which balloon contains the greatest mass of gas? Explain. (b) Compare the average kinetic energies of the gas molecules in the balloons. Explain.
Lecture 6 - Ideal gas law, rising and sinking air The ideal gas law equation tells us that the pressure of the air in the balloon will increase. The increase is momentary though. Because the pressure inside is now greater (the big yellow arrows) than the pressure outside, the balloon will expand. As volume begins to increase, the pressure of the air inside the balloon will decrease.
A sample of nitrogen gas is collected - Mrs. Behymer Chemistry One way to increase the volume of the. gas in the balloon in the diagram. above is to — F . cool the gas in the balloon only. G . increase the temperature of the water _ H . push the balloon farther down into the. water bath. J . seal the top of the water bath. One of the main assumptions of the. kinetic molecular theory of gases is. that the ...
Ideal Gas Law Questions and Answers - Study.com Calculate the new volume if a gas sample at a volume of 13.3 L, initially at a pressure of 2.51 atm, is subjected to an increase in pressure equivalent to 65.0 mmHg. View Answer
As the temperature of the gas in a balloon decreases If a balloon is squeezed, what happens to the pressure of the gas inside the balloon? One way to increase the volume of the gas in the balloon in the diagram above is to - If a balloon containing 3000 l of gas at 39 You place a balloon in a closed chamber at standard temperature and pressure
If a balloon is squeezed, what happens to the pressure of ... One way to increase the volume of the gas in the balloon in the diagram above is to - As the temperature of the gas in a balloon decreases, which of the following occurs? If a balloon containing 3000 l of gas at 39 You place a balloon in a closed chamber at standard temperature and pressure
DOCX Quiz Standard 4 Answer Key - Richmond County School System The balloon is going to look somewhat deflated to due to the decrease in volume. Temperature and gas volume are directly proportional, so if one decreases so does the other. But there will always be some volume so it could not have been completely deflated.
Exampro GCSE Biology - Mount Grace School Q1.€€€€€€€€€ The diagram shows the mass of carbon exchanged between carbon reservoirs and the ... €€€€ Suggest one way in which a grower could increase the yield of tomatoes from plants ... Gas collects in the balloon. The gas is then released through the valve and is burned at the
Boyle's Law - Definition, Equation, & Facts with Examples This equation can be used to predict the increase in the pressure exerted by a gas on the walls of its container when the volume of its container is decreased (and its quantity and absolute temperature remain unchanged). Examples of Boyle's Law. When a filled balloon is squeezed, the volume occupied by the air inside the balloon decreases.
As the temperature of the gas in a balloon decreases which of ... One way to increase the volume of the gas in the balloon in the diagram above is to - If a balloon containing 3000 l of gas at 39; An ideal gas differs from a real gas in that the molecules of an ideal gas _____. A real gas differs from an ideal gas because the molecules of real gas have; You place a balloon in a closed chamber at standard ...
Volume and pressure in gases - the gas laws - Temperature ... Volume and pressure in gases - the gas laws Boyle's law. Decreasing the volume of a gas increases the pressure of the gas. An example of this is when a gas is trapped in a cylinder by a piston.
PDF Name Chemistry / / SOL Questions - Chapter 10 5. _____ One way to increase the volume of the gas in the balloon in the diagram below is to - a. cool the gas and the balloon only b. increase the temperature of the water c. push the balloon farther down into the water bath d. seal the top of the water bath 6. _____ One of the main assumptions of the kinetic molecular theory
PDF Chem. 100 Experiment Name: Chemistry 100 Laboratory ... The temperature and volume of a gas are directly proportional. If the temperature of a gas is doubled then the volume is doubled if the pressure remains the same. V 1 = V 2 T 1 T 2 Pressure Standard pressure is 1 atmosphere (atm) or 760 mm Hg Temperature When doing gas calculations the Celsius temperature must be changed to Kelvin.
One way to increase to volume of the gas in the balloon ... If the volume of gas in the balloon remains constant, then an increase in temperature would result in an increased gas pressure in a balloon.That result can be achieved in three ways:1). Pump more...
Unit 1 Review: Properties of Matter Quiz - Quizizz Q. An experiment was performed to determine the specific heat of some common substances. Using the information in the data table above, choose the most reasonable conclusion. answer choices. Metals require more energy than nonmetals to raise the temperature 1 k. Lead requires the most energy to raise its temperature 1k.

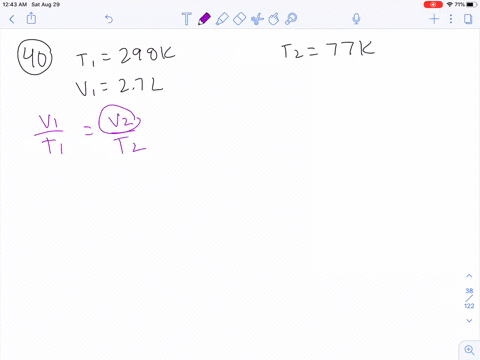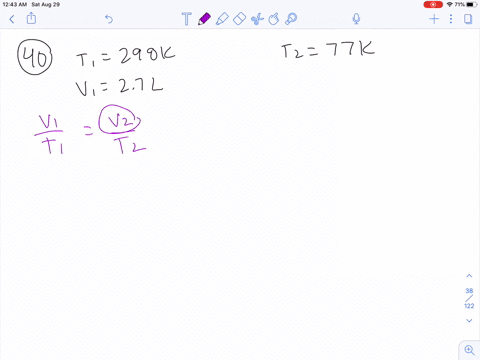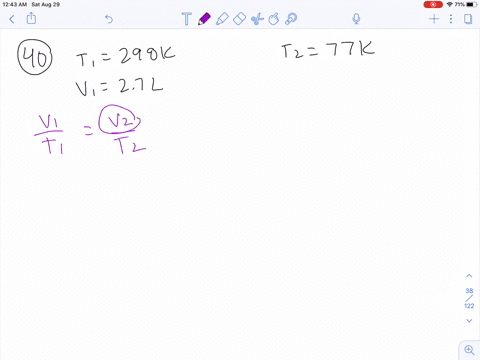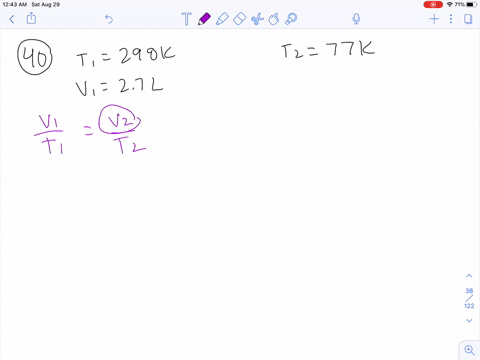








/GettyImages-91560144-56a133d53df78cf772685abd.jpg)



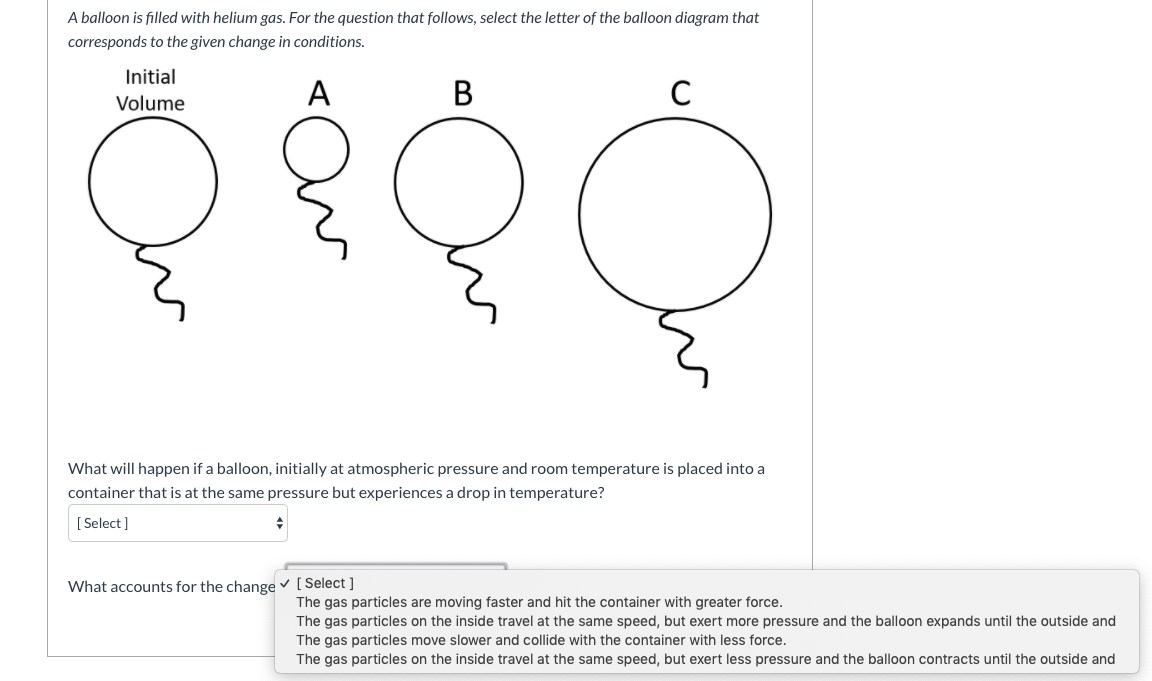








0 Response to "45 one way to increase the volume of the gas in the balloon in the diagram above is to -"
Post a Comment