41 lewis dot diagram h2o
How can I draw the Lewis structure for H2O? | Socratic For H₂O, O must be the central atom The skeleton structure is H-O-H. O has 6 valence electrons, and each H has one. You must arrange 8 electrons in pairs so that O has 8 and each H has two electrons in its valence shell. The trial structure is You have eight valence electrons in your trial structure, so it has the correct number of electrons. issrmaterecclesiae.it Feb 17, 2022 · Lewis Dot Structures The Lewis dot diagram for iodite (IO2-) is A quick and simple explanation for drawing the electron dot diagram for SCl6 This molecule is also known by the name Acetylene The bigger lobe of the hybrid orbital always has a positive sign, while the smaller lobe on the opposite side has a negative sign The bigger lobe of the A quick and …
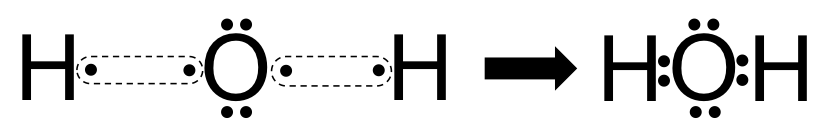
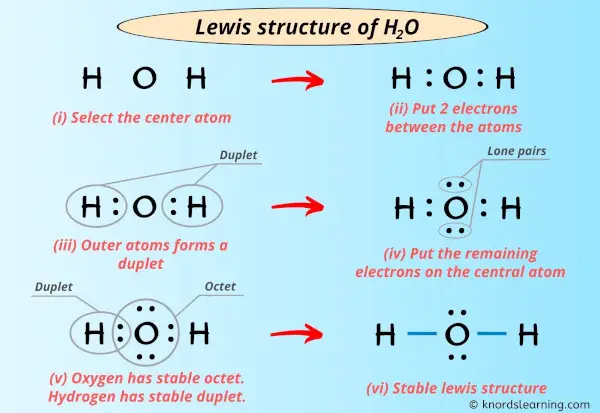
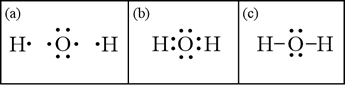
What is the Lewis dot structure for H2O? - Answers The Lewis dot structure of water begins with a single O atom in the center. On the right and left sides are a singly bonded H atom. The O atom then has one pair of dots on the unbonded sides.

Lewis dot diagram h2o
What's what's the Lewis structure for 2H2O Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. In the Lewis-dot structure the valance electrons are shown by 'dot'. The representation of valence electrons of an atom by dots around the symbol of an element is said to be the Lewis-dot symbol. (PDF) Engineering Chemistry by Jain & Jain | Inder Rahi Academia.edu is a platform for academics to share research papers. How to find the Lewis dot diagram for H2O - Quora Answer (1 of 2): Firstly you need to know the number of electrons present in outermost shell of O and H atom O: 1s2 2s2 2p4 There are six electrons in outermost shell H: 1s1 there is one electron in outermost shell Write down the symbol of atom and no. of electrons in the outermost shell are r...
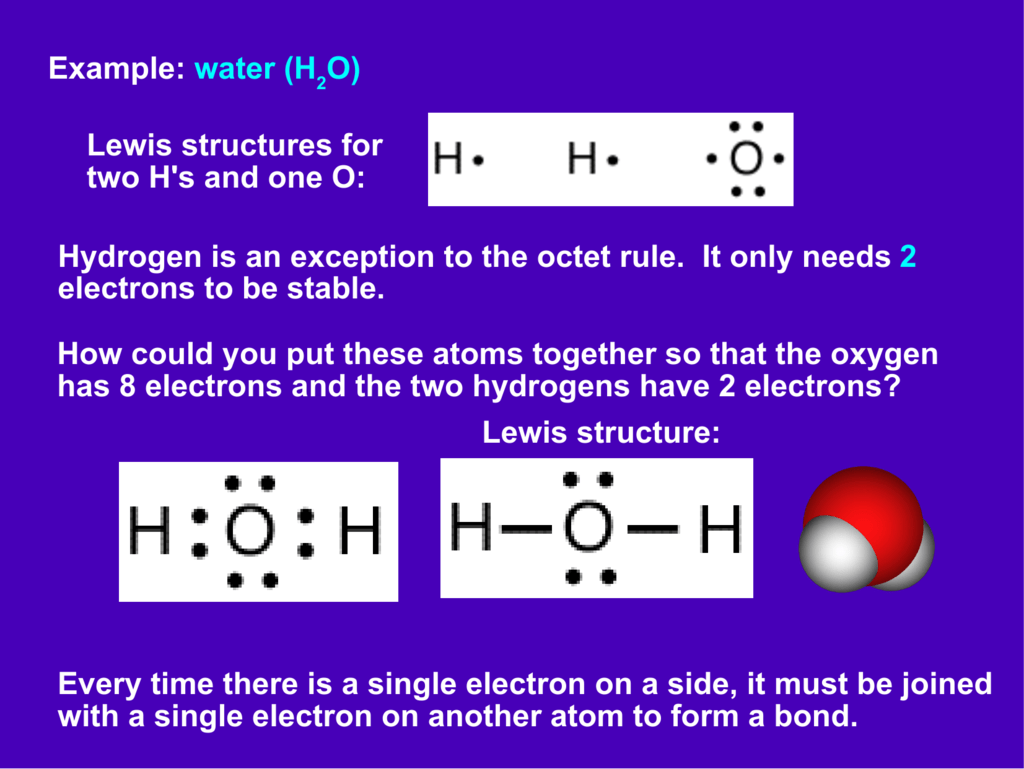
Lewis dot diagram h2o. What is the Lewis dot for h20? - Morethingsjapanese How to Draw a Lewis Dot Structure Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element. What is the Lewis dot diagram purpose? Lewis Dot of Water H2O - Kentchemistry.com 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. They follow the duet rule (2 electrons). Water is a transparent, tasteless, odorless liquid at room temperature and standard pressure. Water is a polar molecules. What is the Lewis dot structure for h2o? - FindAnyAnswer.com Drawing the Lewis Structure for H2O You have a total of 8 valence electrons available to fill the octets of Oxygen and Hydrogen. Remember that Hydrogen only needs two electrons to have a full outer shell. It is helpful if you: Try to draw the H2O Lewis structure before watching the video. What is the structural formula of water? H2O How do you draw a Lewis dot structure for water? Answer and Explanation: Water, or H2O H 2 O , has the electron dot structure shown below. The structure must have a total of 8 valence electrons because there are 2 Subsequently, question is, what type of bond is water? Water is a polar molecule A water molecule is formed when two atoms of hydrogen bond covalently with an atom of oxygen.
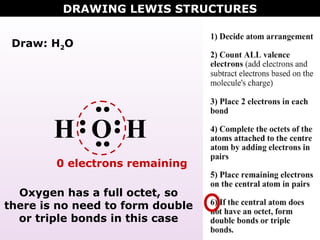
Lewis Structure of H2O (Water) - Drawing Steps H 2 O lewis structure In the lewis structure of H 2 O, there are two single bonds around oxygen atom. Hydrogen atoms are joint to oxygen atom through single bonds. Also, there are two lone pairs on oxygen atom. Water molecule is a simple molecule. Drawing lewis structure of water molecule is simple than some of other complex molecules or ions. H2O Molecular Geometry, Lewis Structure, Shape and Bond Angles This is the Lewis structure of the H2O molecule that has two single bonds between Oxygen and Hydrogen. As a result, there are two lone pairs in this molecule and two bonding pairs of electrons. H2O Hybridization When two atoms share electrons and form bonds, there is the formation of hybridized orbitals. Answered: A proportion of fentanyl is known to be… | bartleby Mar 27, 2022 · A proportion of fentanyl is known to be lost to IV infusion bags made from PVC on storage and this effect is known to be pH dependent. Explain why you believe this effect occurs and what potentially could be done to minimise loss of fentanyl to the PVC packaging Lewis Dot Diagram Of Nh3 Water (H2O) Lewis Dot Structure. by crator-avatar Patricia Andrade 0. ; 0; 3. NH3 (Ammonia) is a commonly tested Lewis structure. It's not In the NH3 Lewis structure (and all structures), hydrogen goes on the outside. Remember, too. Step method to draw lewis structure of ammonia. Step 1: Find valence Alternatively a dot method can be used to ...
Lewis dot diagrams for the following:(a) Hydrogen (H2) (b ... Lewis dot diagrams for the following: (a) Hydrogen (H 2 ) (b) Water (H 2 O) (c) Carbon dioxide (CO 2 ) (d) Methane (CH 4 ) Medium Solution Verified by Toppr Was this answer helpful? 0 0 Lewis Electron Dot Structures - Detailed Explanation with ... Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures. Lewis Dot Diagram For H2o2 - schematron.org Lewis dot structure of H?? what is the lewis dot structure of H2O2??? Follow. 2 answers 2. Report Abuse. draw the diagram of H2, the diagram of O2 then connect them where it should be connected. IamCat · 1 decade ago. schematron.org: Resolved. Mar 11, · In the chemical formula of hydrogen peroxide(H2O2) why does both the 2 does not get ... topblogtenz.com › nacl-lewis-dot-structure-polarSodium chloride (NaCl) lewis dot structure, polar or nonpolar ... The lewis dot structure of NaCl contains one positive charge on sodium metal and one negative charge on chlorine nonmetal. We have to represent them by putting brackets around them. The lewis diagram of an ionic compound is formed with a different approach than the normal procedure we used for drawing the compounds like NH3, BF3, BrF5, etc.
Lewis Dot Structure for H2O | Chemical Bonding | Success ... Watch the video of Dr. B. drawing the Lewis dot structure for H2O and answer the questions below. The H 2 O Lewis dot structure is seen fairly frequently. A common error it to put two oxygen atoms and one hydrogen making HO 2. Make sure you have two hydrogens and one oxygen in H 2 O! Hint: look at the questions before watching the video.
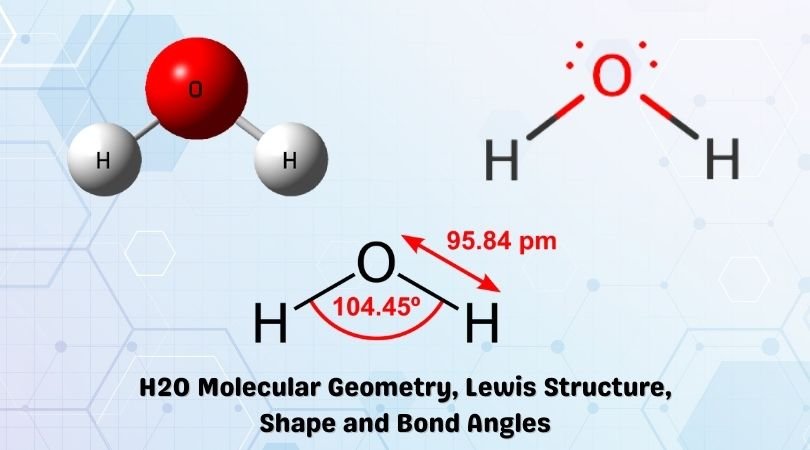
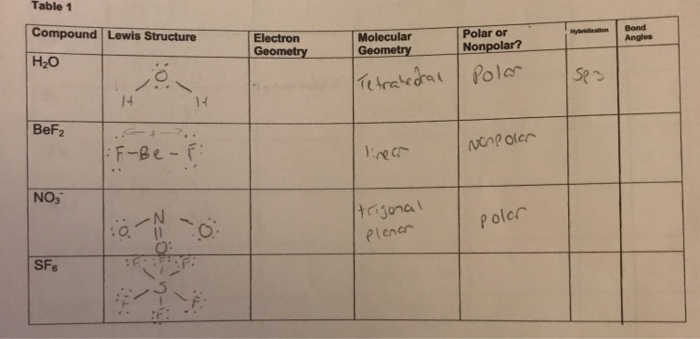
The correct electron dot structure of water molecule class ... So, there is 1 valence electron available for hydrogen in order to form a chemical bonding. In the diagram, provided above we get an idea about the structure of water molecules. Oxygen atom has 6 valence electrons out of which 2 valence electrons are involved in bonding with the 2 hydrogen atoms. Therefore, a water molecule has 2 bond pairs of...
How do you find the Lewis dot diagram for H2O? - Quora O atom need 2 more electron for completely filled outermost L shell while each H atom need 1 more electron for completely filled outermost K shell. So O will ...2 answers · 0 votes: Firstly you need to know the number of electrons present in outermost shell of O and H atom ...
› 49212961 › Inorganic_Chemistry_by(PDF) Inorganic Chemistry by Miessler ~ 5th Edition | Arnab ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry.
Lewis Dot Diagram H2o - schematron.org This is the Lewis Dot Structure for H2O. You could alternatively also draw the structure by including two dots for every bond. While oxygen's octet seems to have.The Lewis dot structure of water begins with a single O atom in the center. On the right and left sides are a singly bonded H atom.
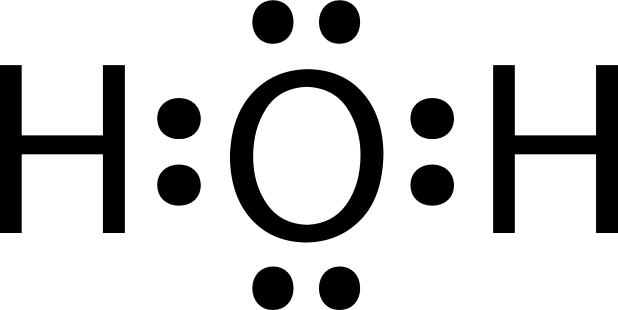
Lewis structure - Simple English Wikipedia, the free ... Lewis structure of water. The Lewis structures, also called Lewis-dot diagrams or electron dot diagrams or Lewis dot structure are pictures that show the bonding between a pair of electrons and the atoms of a molecule . Each dot represents one electron. Two dots side by side represent a lone pair of electrons.
lewis structure for h2o water | H2O Molecular Geometry ... H2O's Lewis Dot Structure gives it many unique properties mostly due to the two lone pairs on the central oxygen atom. This increases electron-electron repulsion and therefore creates a bent structure as opposed to CO2's linear structure.
Covalent bond and Lewis dot structure (H2O & CO2) (video ... Covalent bond and Lewis dot structure (H2O & CO2) Google Classroom Facebook Twitter. Email. Bonding in carbon- covalent bond. Carbon and hydrocarbons. Covalent bond . Covalent bond and Lewis dot structure (H2O & CO2) This is the currently selected item. Single and multiple covalent bonds.
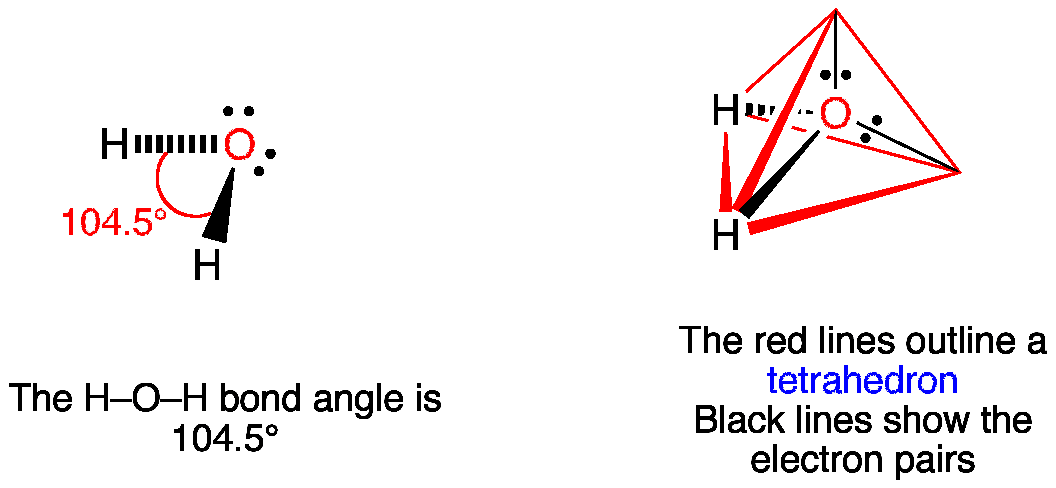
H2O Lewis Structure, Molecular Geometry, and Hybridization Draw the lewis diagram: The Geometrical Structure of the H2O molecule The bond angle among hydrogen-oxygen-hydrogen atoms (H-O-H) is 104.5°. From this, it can be understood that the geometrical structure of a single H2O molecule is bent.
issuu.com › osanoothu › docsDoc 117 b p s xi chemistry iit jee advanced study ... - Issuu Sep 05, 2016 · BRILLIANT PUBLIC SCHOOL, SITAMARHI (Affiliated up to +2 level to C.B.S.E., New Delhi) Class-XI IIT-JEE Advanced Chemistry Study Package Session: 2014-15 Office ...
Water Lewis Structure - How to Draw the Lewis Structure ... A step-by-step explanation of how to draw the H2O Lewis Dot Structure (Water).For the H2O structure use the periodic table to find the total number of valenc...
Lewis Dot Diagram H2o The Lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. The other four valence electrons in oxygen are in pairs at the bottom. The Lewis dot structure of water begins with a single O atom in the center. On the right and left sides are a singly bonded H atom.
› category › celebritiesCelebrities Archives - Hollywood.com Hollywood.com is your place to hear what's good with of your favorite Hollywood celebrities. No gossip, no negative news, no paparazzi photos.
Draw Lewis dot diagram for the following. Water (H2O ... Draw Lewis dot diagram for the following. Water (H2O) Maharashtra State Board HSC Science (Electronics) 11th. Textbook Solutions 6926. Important Solutions 18. Question Bank Solutions 4571. Concept Notes & Videos 336. Syllabus. Advertisement Remove all ads. Draw Lewis dot diagram for the following. ...
chem Flashcards - Quizlet The Lewis dot structure is used to keep track of the valence electrons for each atom. Give the symbol of the element in Group 6A, Period 3. S. Write the symbol for the element with the following electron configuration? 1s22s22p63s23p1. Al. Write the symbol for the element with the following electron configuration. [Kr]4s23d6. Fe. Match the type of …
(PDF) Inorganic Chemistry 4th edition, Catherine Housecroft Inorganic Chemistry 4th edition, Catherine Housecroft
Lewis Dot Structure of H2O, (Water) - YouTube I quickly take you through how to draw the Lewis Structure of water, H2O. I also go over hybridization, shape and bond angle.
What is the Lewis dot structure of H2O? - Answers The Lewis dot structure of water begins with a single O atom in the center. On the right and left sides are a singly bonded H atom. The O atom then has one pair of dots on the unbonded sides.
Solved Draw a Lewis dot structure for water and 4 other ... Transcribed image text: Draw a Lewis dot structure for water and 4 other water molecules surrounding that water molecule with hydrogen bonds to the central structure. ty, Solutions and Rates of Chemical Prelab Experiment #7 1. 2. Briefly explain how detergent is able to get nonpolar substances, such as oil, to mix with water in order to clean clothes or dishes.
Lewis acids and bases - Wikipedia A center dot may also be used to represent a Lewis adduct, such as Me 3 B·NH 3. Another example is boron trifluoride diethyl etherate, BF 3 ·Et 2 O. In a slightly different usage, the center dot is also used to represent hydrate coordination in various crystals, as in MgSO 4 ·7H 2 O for hydrated magnesium sulfate, irrespective of whether the water forms a dative bond with the …
What is the Lewis structure of H2O? | Socratic Oct 28, 2015 — It should look like this with the single line representing the sharing of two electrons between them.1 answer · Explanation: It should look like this with the single line representing the sharing of two electrons ...
Water Lewis Structure: How to Draw the Dot Structure for ... Drawing the Lewis Structure for Water Viewing Notes: Make sure you put the correct atom at the center of the Water (H 2 O) molecule. With the Lewis Structure for Water (H 2 O) remember that water only needs two valence electrons to have a full outer shell. Be sure that you don't use more than the eight valence electrons available.
MakeTheBrainHappy: The Lewis Dot Structure for H2O H2O's Lewis Dot Structure gives it many unique properties mostly due to the two lone pairs on the central oxygen atom. This increases electron-electron repulsion and therefore creates a bent structure as opposed to CO2's linear structure. This "bent" molecular structure gives it many unique properties such as being polar.
How to find the Lewis dot diagram for H2O - Quora Answer (1 of 2): Firstly you need to know the number of electrons present in outermost shell of O and H atom O: 1s2 2s2 2p4 There are six electrons in outermost shell H: 1s1 there is one electron in outermost shell Write down the symbol of atom and no. of electrons in the outermost shell are r...
(PDF) Engineering Chemistry by Jain & Jain | Inder Rahi Academia.edu is a platform for academics to share research papers.
What's what's the Lewis structure for 2H2O Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. In the Lewis-dot structure the valance electrons are shown by 'dot'. The representation of valence electrons of an atom by dots around the symbol of an element is said to be the Lewis-dot symbol.






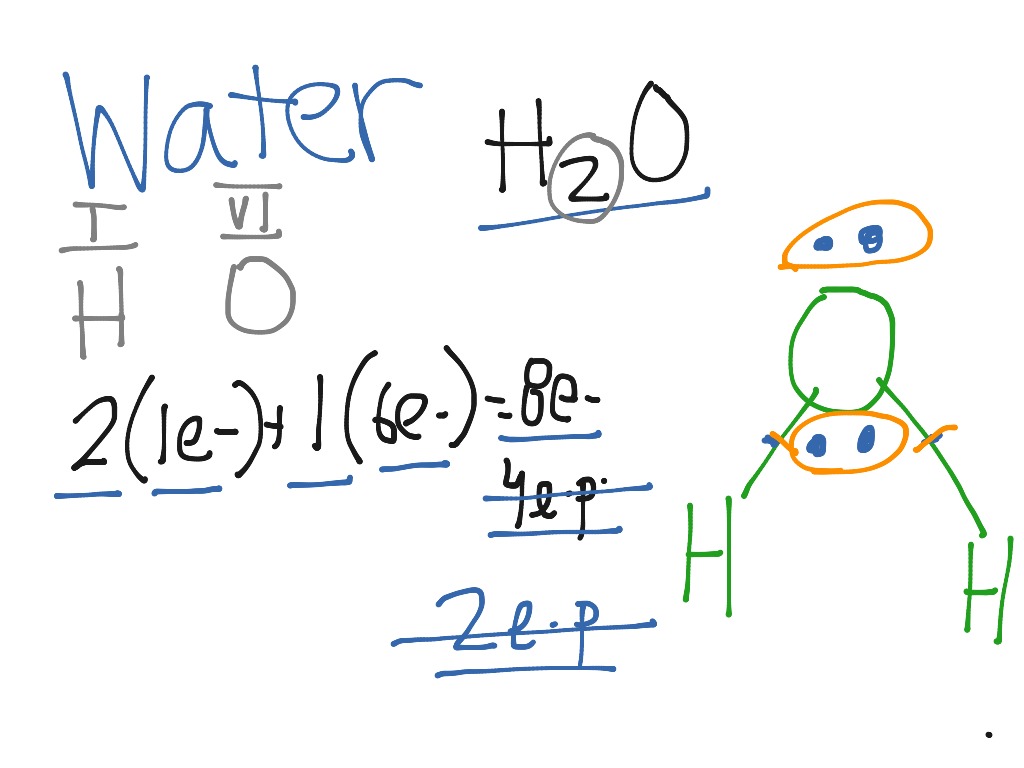


/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)

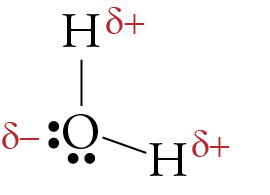












0 Response to "41 lewis dot diagram h2o"
Post a Comment