41 molecular orbital diagram for o2 2
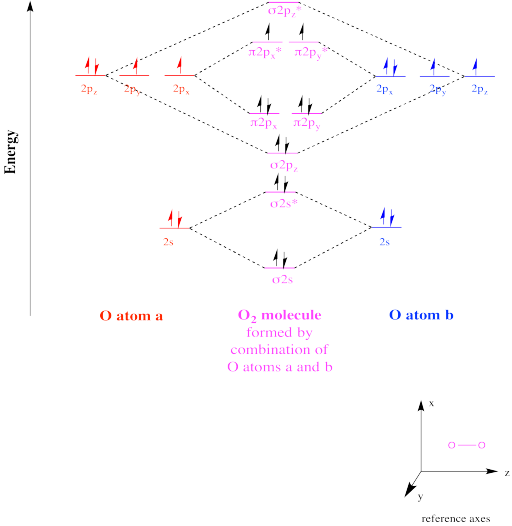
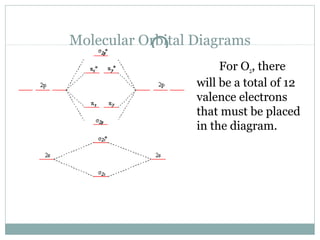
PDF Miessler-Fischer-Tarr5e SM Ch 05 CM O2. Therefore, NF is predicted to be paramagnetic with a bond order of 2. The populations of the bonding (8 electrons) and antibonding (4 electrons) molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold rotation axis. The 2p and 2p * Give the molecular orbital energy diagram of N2 and O2 ... Electronic configuration of Oxygen (Z = 8) is 1s 2 2s 2 2p 4. Since Oxygen atom has 8 electrons, the molecular orbitals of Oxygen molecule (O 2) has 16 electrons, which are distributed as below : Molecular orbital energy level diagram of O 2 • Bond order = (10 - 6)/2 = 2(O = O) • Presence of two unpaired 6 electrons (π * 2p 1 y π* 2p 1 z ...
MO DIAGRAM O2+ , O2 2+ ,O2- ,O2 2- (preparation of gate ... Follow me on instagram- me on facebook page- ...
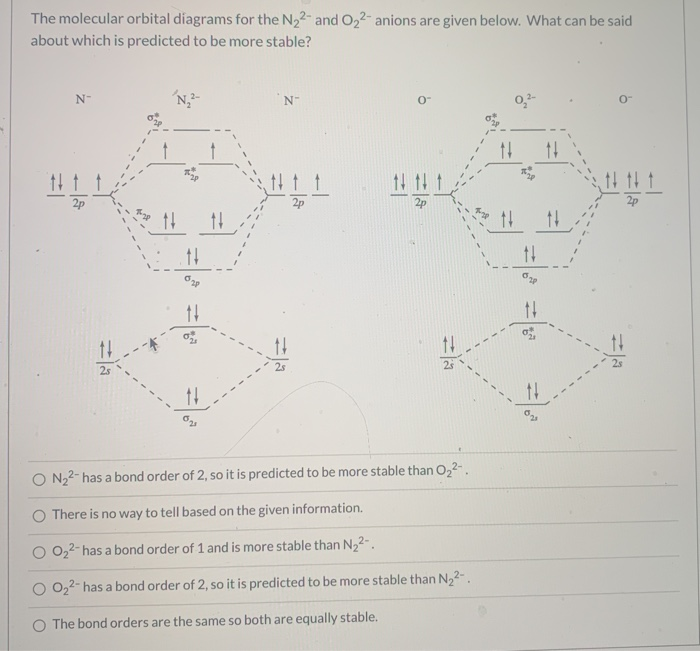
Molecular orbital diagram for o2 2
He2 2+ Molecular Orbital Diagram - schematron.org A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ... MO Diagram for O2(2-) - YouTube It is sigma2s(2)sigma2s*(2)sigma2p(2)pi2p(4)pi2p*(4)Bond order 1. It is stable. In fact, it's the perioxide ion.Check me out: Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
Molecular orbital diagram for o2 2. Draw the molecular orbital energy diagram for oxygen ... Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown: (i) Electronic configuration: (ii) Bond order: Here N b = 8; N a = 4 The two oxygen atoms in a molecule of oxygen are united through two covalent ... Bond order ofO2 O2+ O2 and O22 is in order A O2 langle ... To solve this question, we need to write the molecular orbital configuration. To find out the bond order from the molecular orbital configuration is: Bond order = 1 2 [ Bonding - antibonding] Complete step by step answer: Let's first draw the MOT of the oxygen molecule. The oxygen molecule O 2 contains the 16 electrons. Explain the formation of O2 molecule using molecular class ... We know that Oxygen has atomic number = 8. Thus, the electronic configuration for an atom of oxygen in the ground state can be given as - $1{s^2}2{s^2}2{p^4}$ One atom of oxygen has 8 electrons. Thus, two atoms will possess 16 electrons i.e. Oxygen molecules will have 16 electrons. The molecular orbital diagram of an Oxygen molecule is as - Molecular Orbital (MO) Diagram for O2(2-) - YouTube This is the peroxide ion, O2(2-), so you KNOW it's going to be stable.It has a bond order of 1, which also makes sense. Draw the Lewis diagram of hydrogen pe...
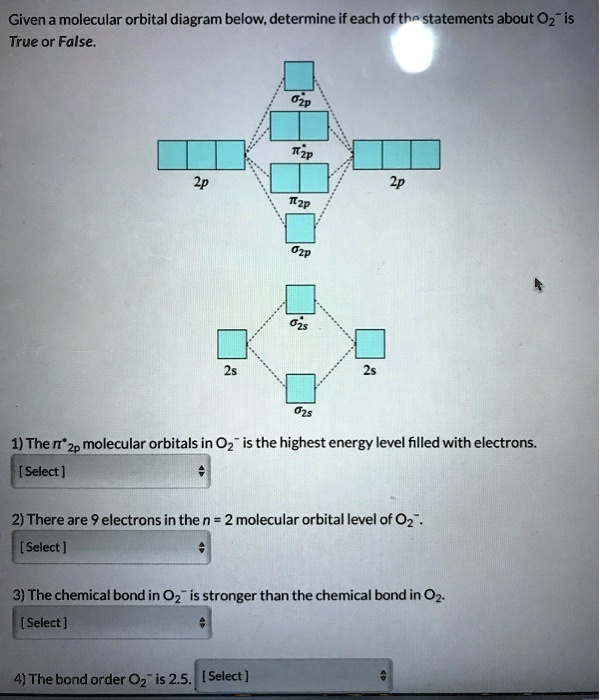
Electronic valence molecular orbital configuration of "O ... You'll need the molecular orbital (MO) diagram of O2. Begin with the atomic orbitals. Oxygen atom has 2s and 2p valence orbitals and 6 valence electrons: Each oxygen contributes 6, so we distribute 12 valence electrons into the molecule to get O2. Two 2s orbitals combine to give a σ2s bonding and σ* 2s antibonding MO. What Is The Molecular Orbital Diagram Of O2 ... What is the bond order of O2 -? 2 O2 has two unpaired electrons in its π* orbitals, and a bond order of 2. How do you draw a molecular orbital diagram for O2? 0:003:38Molecular Orbital (MO) Diagram of O2 - YouTubeYouTube. How many electrons are in bonding orbitals in O2? 1: Molecular Orbital Energy-Level Diagrams for O2. Solved Sketch the molecular orbital (MO) diagram for O2^2 ... Sketch the molecular orbital (MO) diagram for O2^2 -. Is the molecule paramagnetic or diamagnetic? Calculate the bond order. Verify the bond order by drawing the Lewis Structure Is this ion more stable or less stable than elemental oxygen? WHY? Using the table of average bond energies below, calculate Delta H for the reaction below: H-C ... MOLECULAR ORBITAL DIAGRAM OF O2, 02+AND O2(2 ... - YouTube In this video, you will study about Molecular Orbital diagram of O2, O2+, O2(2-). We will also calculate the Bond order in each case and also the magnetic be...
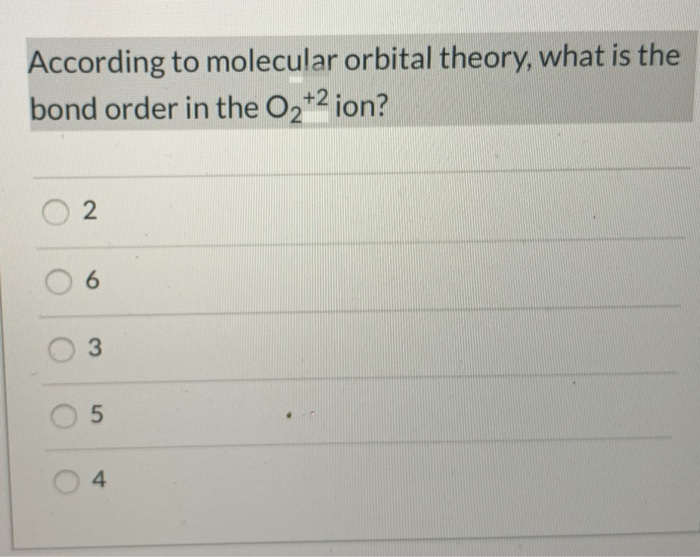
8 - Drawing Molecular Orbital Diagrams — Flux Science Well, s-p mixing doesn't occur with diatomic oxygen, creating a molecular orbital diagram like the first in this article. This is because, as more electrons are added to a system, the higher the energy becomes, due to their electrostatic repulsion. If the energy of the 2s and 2p orbitals are too far apart, mixing won't occur. What's the MOT diagram of O2 +2 ion? - Quora Answer (1 of 3): In O2 2+, there is 14 electrons. So, it's MOT is comparable to N[code ]2[/code] & the MOT diagram will look like this : Draw molecular orbital diagram of O2 or N2 with magnetic ... Draw molecular orbital diagram of O 2 or N 2 with magnetic behavior and bond order. Medium Solution Verified by Toppr As it can be seen from the MOT of O 2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b −N a ]/2=[10−6]/2=2. molecular orbital diagram of o2 o2- o2+ - Brainly.in Molecular orbital diagram of o2 o2- o2+ 1 See answer Advertisement Advertisement topper2349 is waiting for your help. Add your answer and earn points. Brainly User Brainly User In chemistry, a molecular orbital (MO) is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate ...
In molecular orbital diagram for O2+ ion the highest class ... Hint: Molecular orbital theory was put forward by Hund and Mulliken, which can be applied to explain the properties, that was not explained by Valence bond theory. This theory explained the paramagnetic nature of O 2 + ion as per Valence bond theory it should be diamagnetic. - Molecular orbital diagram is the diagrammatic representation of all ...
Draw a molecular orbital diagram of N2 or O2 with magnetic ... Molecular orbital theory is a method for describing the electronic structure of the molecule. Now, let us draw the molecular orbital diagram of ${N_2}$ . Now, first let us understand what magnetic behavior and bond order means.
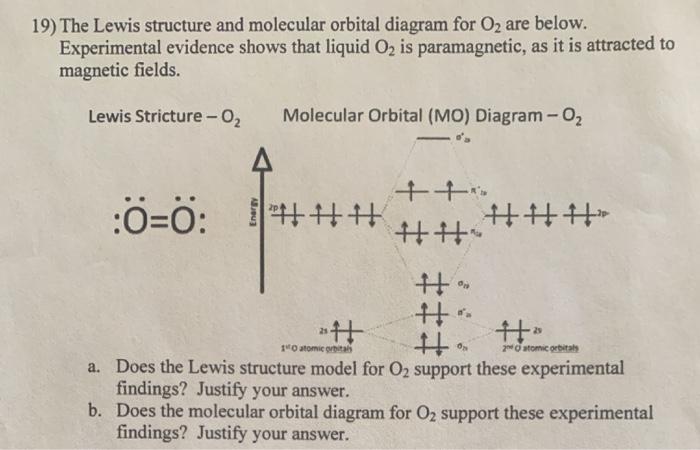
Molecular Orbital Theory - Chemistry Oxygen's paramagnetism is explained by the presence of two unpaired electrons in the (π 2py, π 2pz)* molecular orbitals. Check Your Learning The main component of air is N 2. From the molecular orbital diagram of N 2, predict its bond order and whether it is diamagnetic or paramagnetic.
Molecular Orbital Diagram For Cl2 - schematron.org % (2 ratings). This is the molecular orbital diagram for the homonuclear diatomic Be2+, showing the molecular orbitals of the valence shell only. The molecular orbitals are. The Lewis dot structure famously predicts the wrong electronic structure for O2.
Molecular Orbital (MO) Diagram for O2(2+) - YouTube Remember: When two oxygen atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals. They are flipped compare...
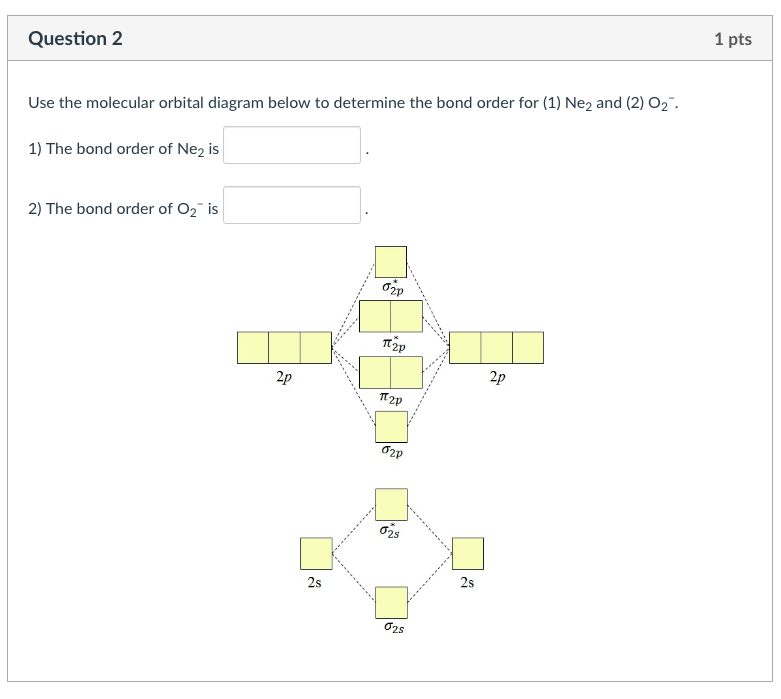
39 molecular orbital diagram for o2 2- - Diagram For You Draw molecular orbital diagram of O2 - - or N2 - Toppr As it can be seen from the MOT of O 2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b − N a ] / 2 = [1 0 − 6] / 2 = 2.
Solved Draw molecular orbital diagrams for O2-, O22-, and ... Draw molecular orbital diagrams for O2-, O22-, and O2. Which has the highest bond order? Which would be paramagnetic, and which would be diamagnetic? Can you draw good dot structures that correspond to each of these ions or molecules? Draw a molecular orbital diagram lor Arz*. This ion has been observed in the gas phase.
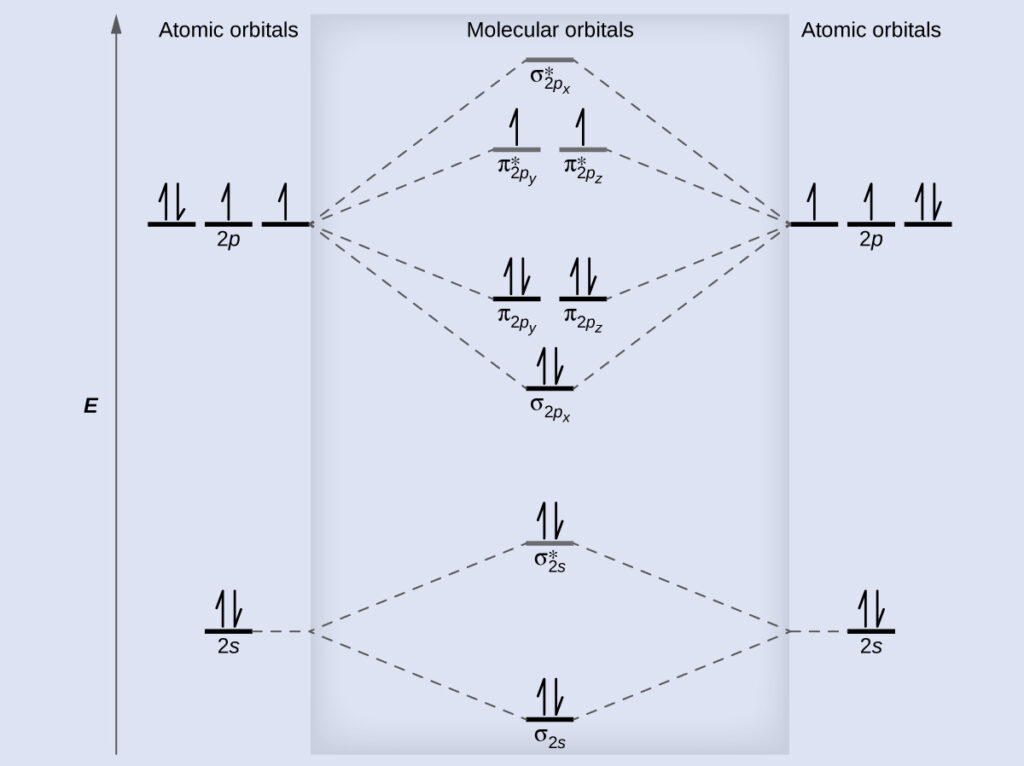
Molecular Orbital Theory - Purdue University The molecular orbital diagram for an O 2 molecule would therefore ignore the 1 s electrons on both oxygen atoms and concentrate on the interactions between the 2 s and 2 p valence orbitals. Molecular Orbitals of the Second Energy Level
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
MO Diagram for O2(2-) - YouTube It is sigma2s(2)sigma2s*(2)sigma2p(2)pi2p(4)pi2p*(4)Bond order 1. It is stable. In fact, it's the perioxide ion.Check me out:
He2 2+ Molecular Orbital Diagram - schematron.org A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ...
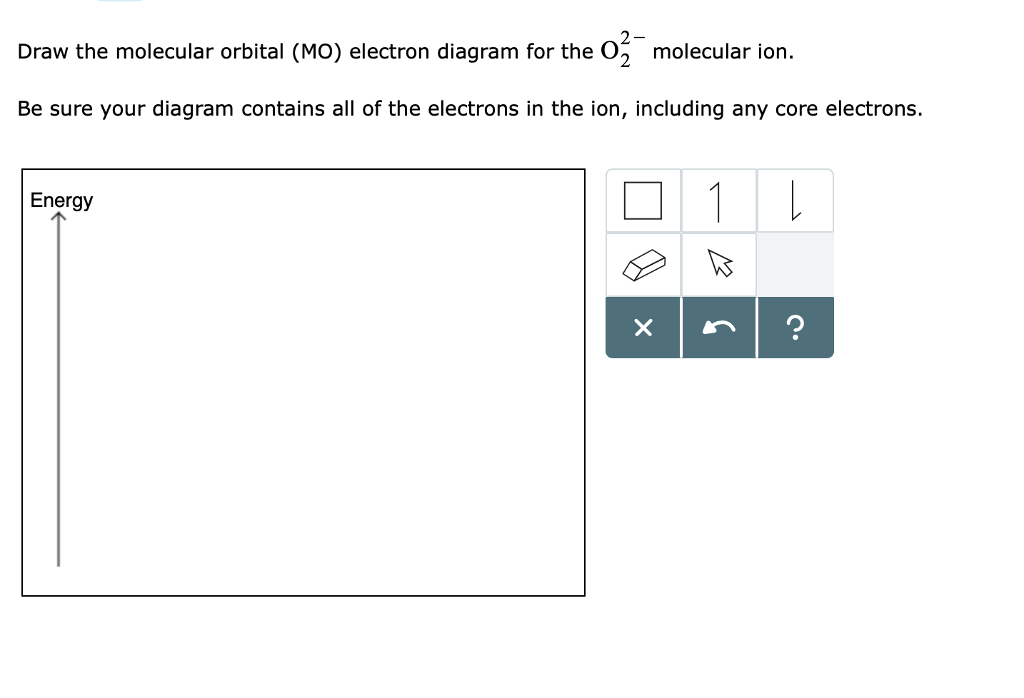
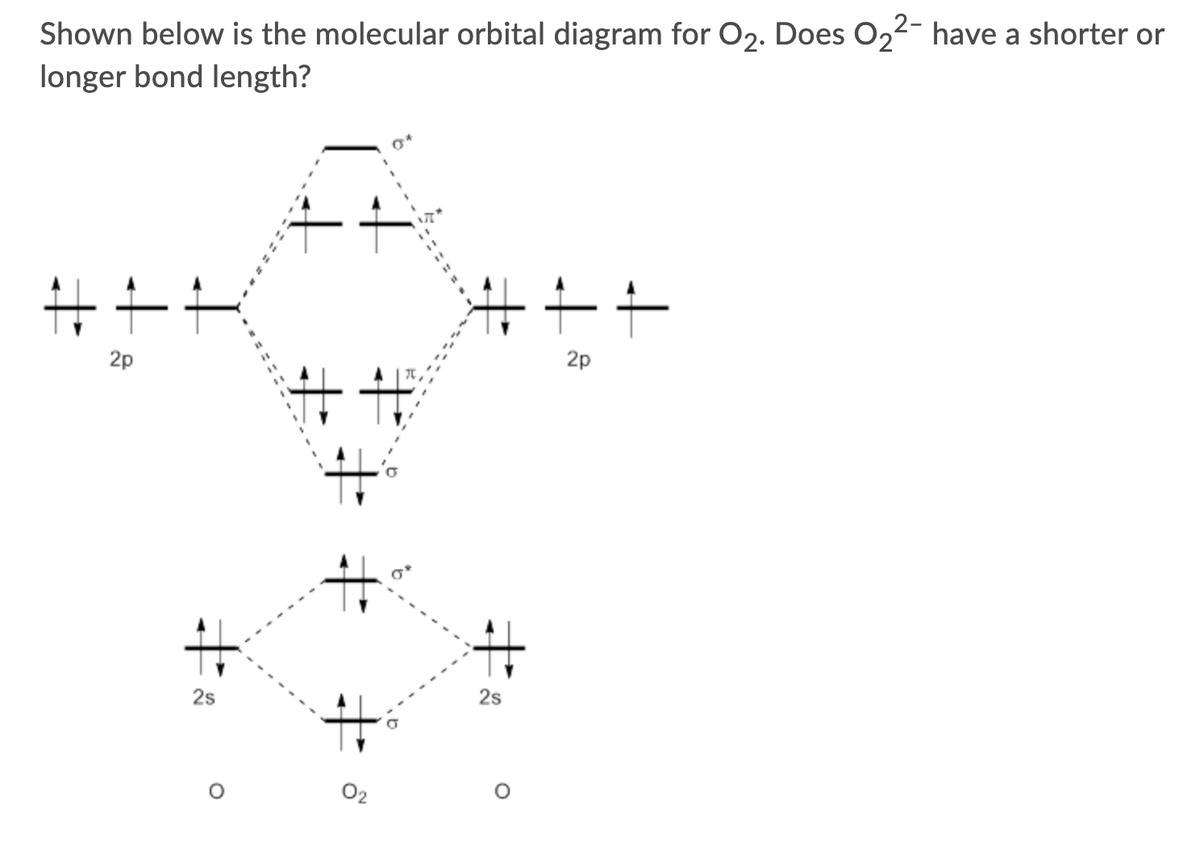



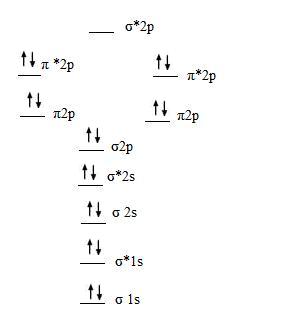





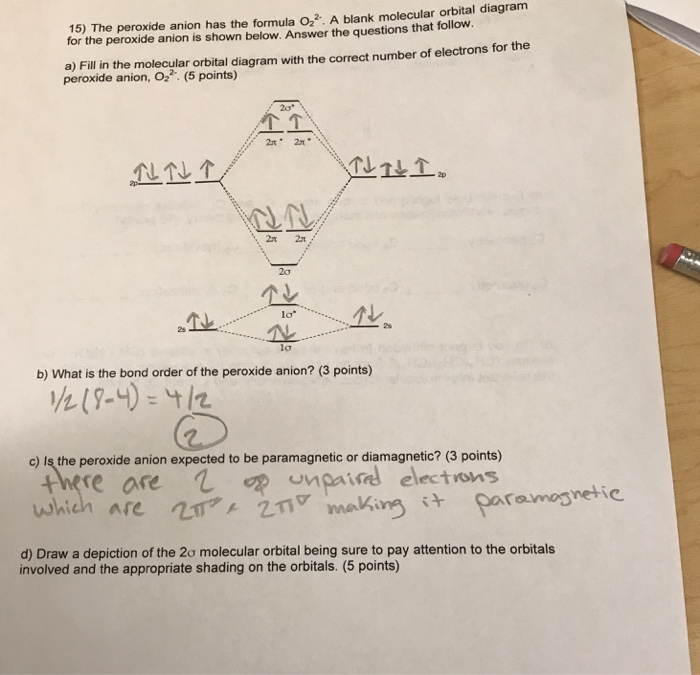
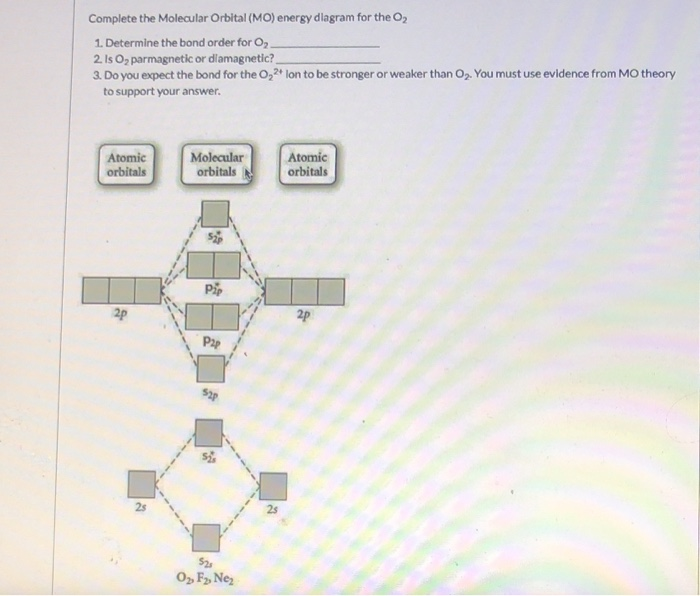
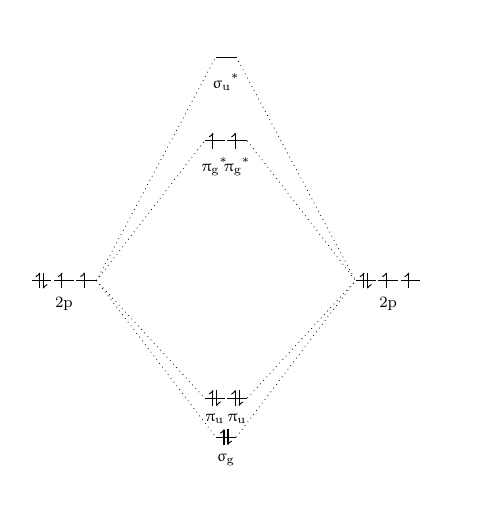
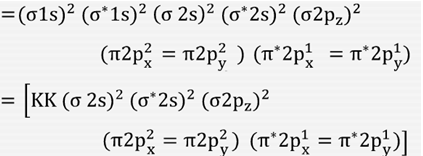










0 Response to "41 molecular orbital diagram for o2 2"
Post a Comment