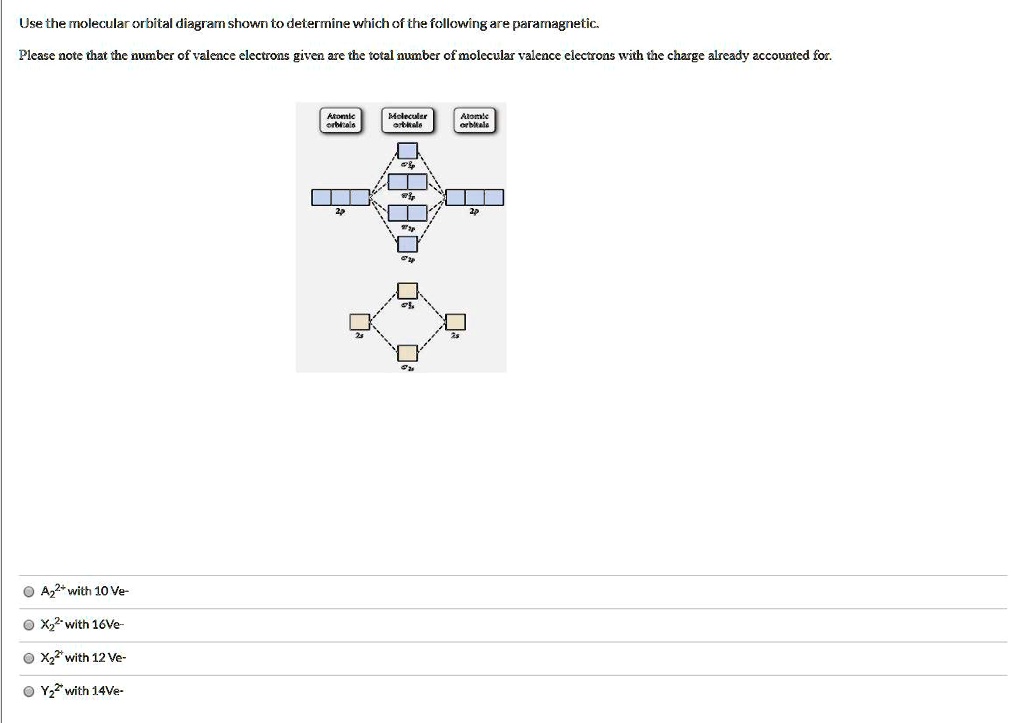
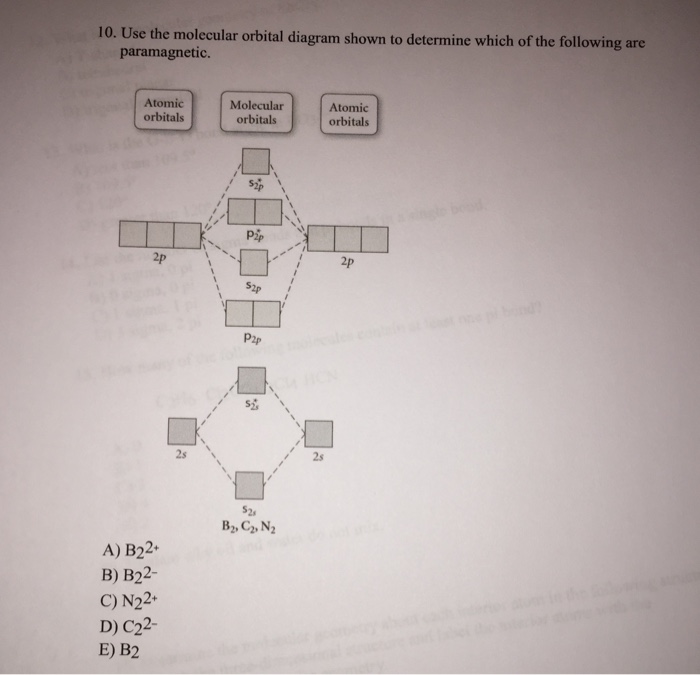
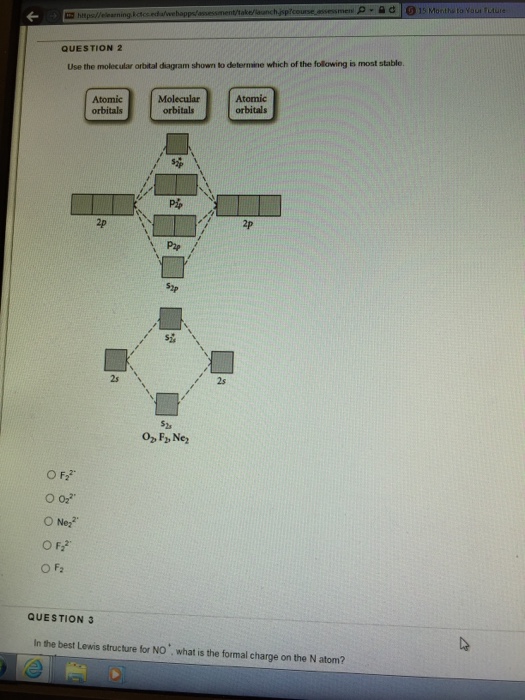
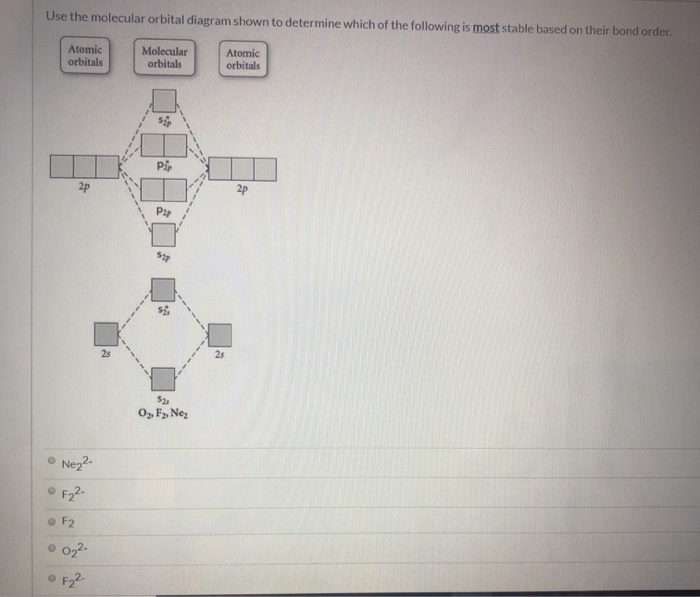
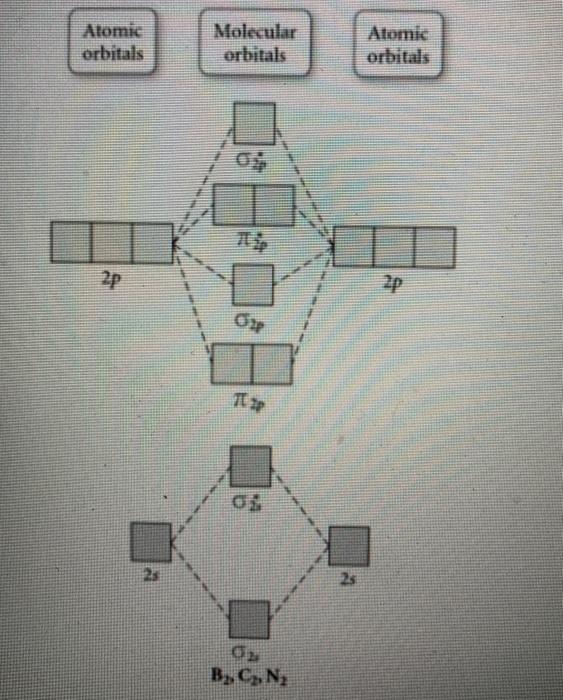
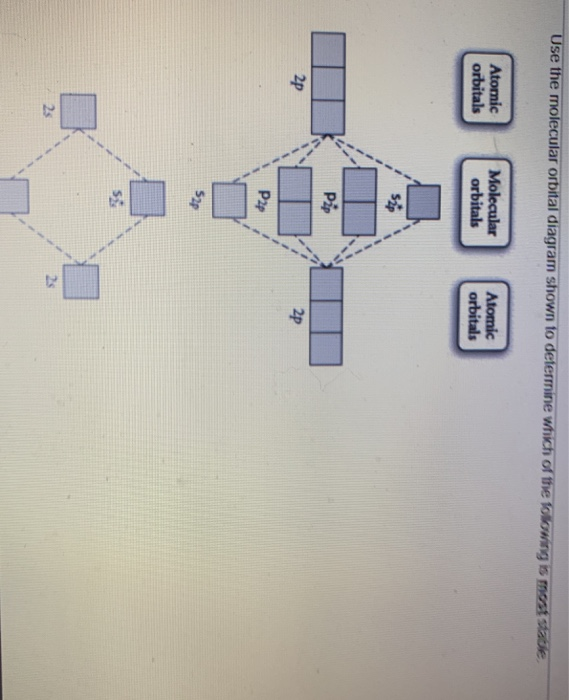
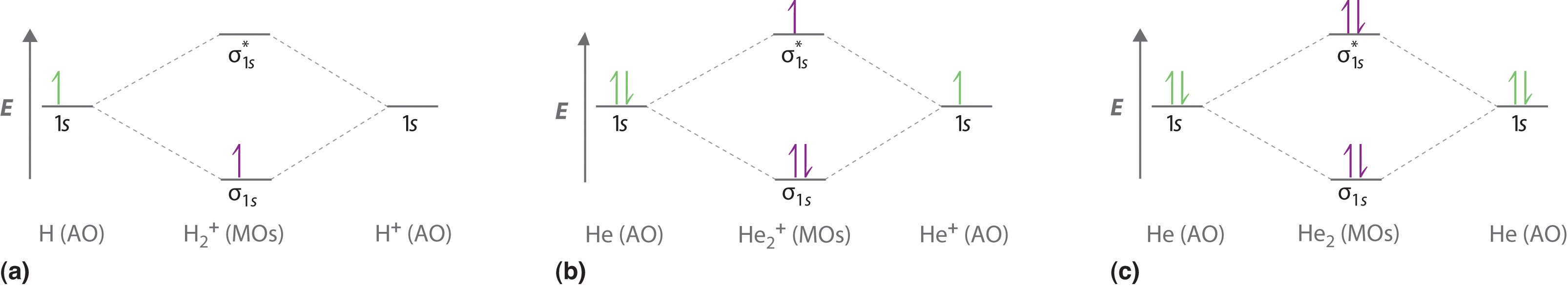
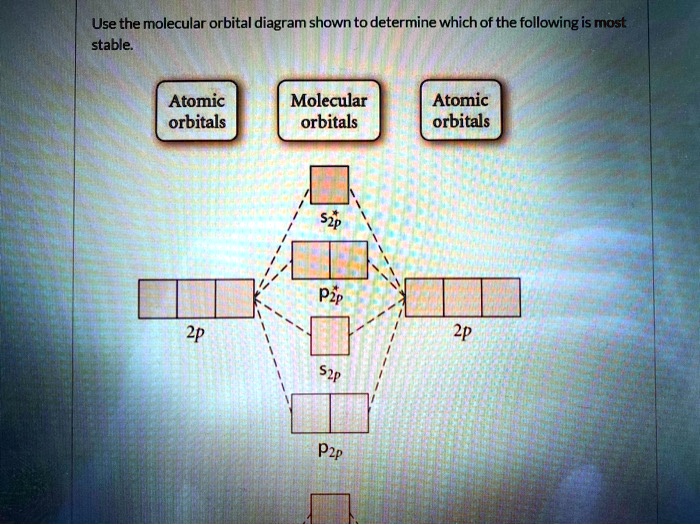
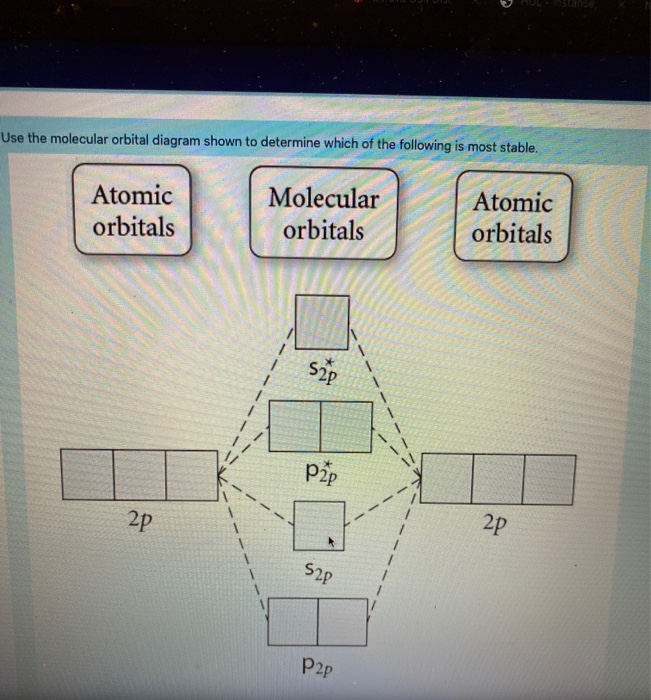
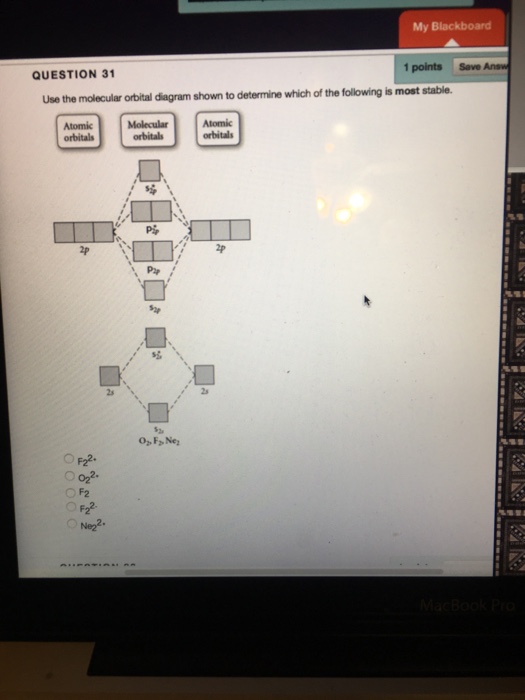
43 draw the molecular orbital diagram shown to determine which of the following is most stable.
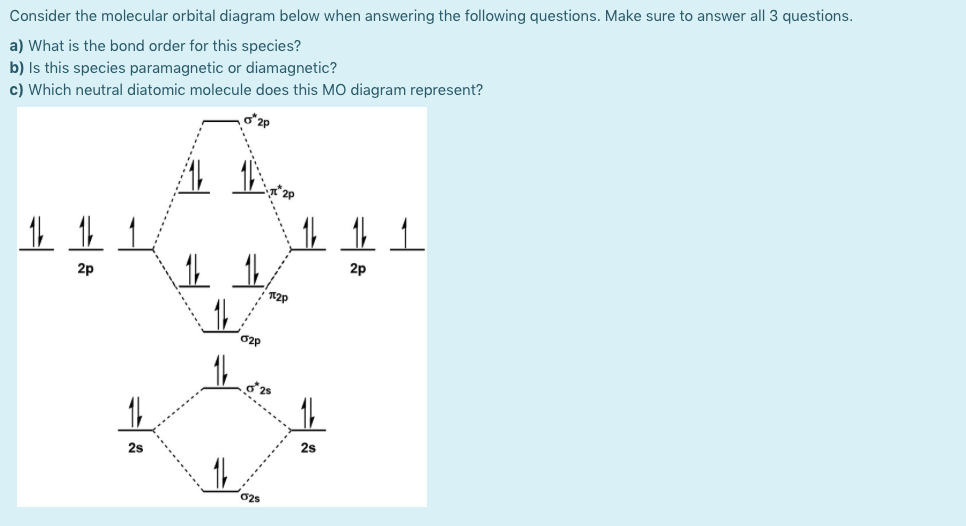
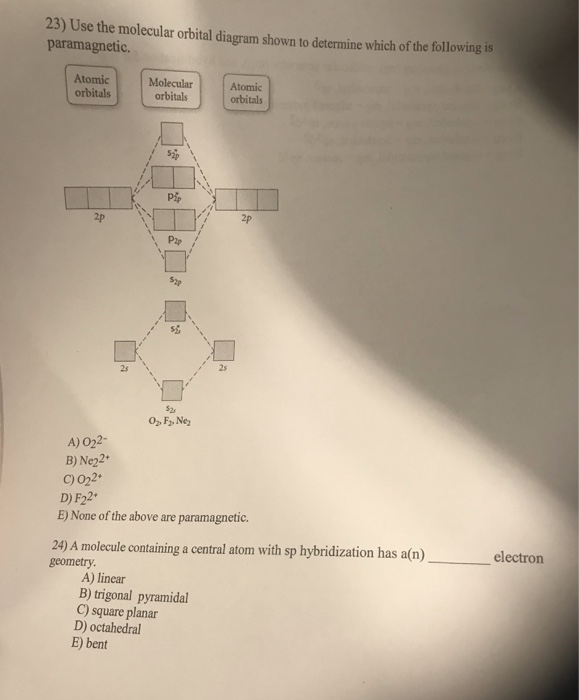
practice orbital diagram Molecular worksheet [7B3YMN] molecular orbital theory build f2 video molecular orbital theory build f2 for the ion f2 a draw the molecular orbital diagram b calculate the bond order c would this ion exist Orbital Diagram For Oxygen - Quiz & Worksheet Practice Drawing Electron Orbital Diagrams Dec 18, 2019 · This video shows a variety of practice problems to determine ... 40 draw the molecular orbital (mo) electron diagram for ... Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
Problem #10: Formation and Properties of Matter [Solved ... In drawing a Lewis diagram of a molecule or an ion, the following rules are followed: Determine the number of valence electrons of each atom in the molecular formula. When the species is an ion, each negative charge counts for 1 electron, whereas each positive charge counts for -1 electron.

Draw the molecular orbital diagram shown to determine which of the following is most stable.
ClO3- Lewis Structure, Molecular Geometry, Hybridization ... The three oxygen atoms take in 3 pairs each and the final pair is marked by Chlorine as shown in the figure. To determine the most stable Chlorate (ClO 3 -) structure, we use the concept of formal charges. Formal charges for an element/structure help determine its most stable Lewis Structure state. C2 Lewis Structure According to the molecular orbital diagram of the C22 ion you its a stable ion because it has a bond order of 1 that means its a stable substance. Electrons are shown as dots or for bonding electrons as a line between the two atoms. Because C 2 H 2 molecule is a simple molecule those all steps may not be used. 38 molecular orbital diagram for ne2 2+ - Wiring Diagrams ... A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular…
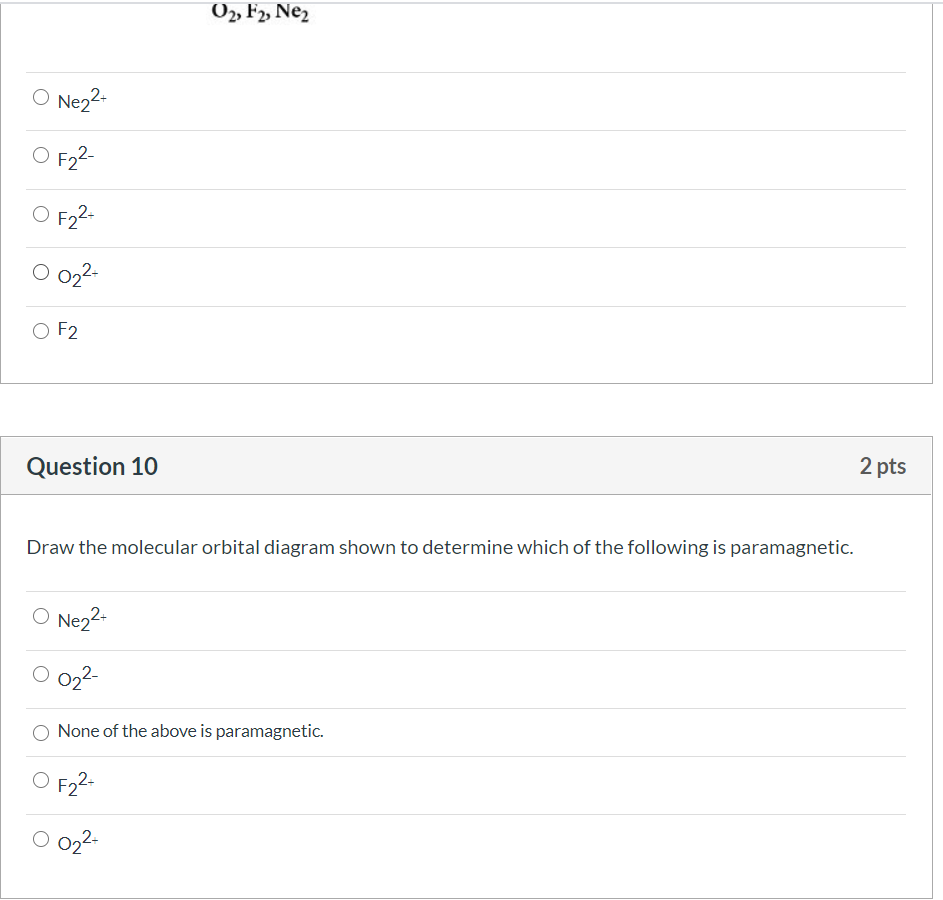
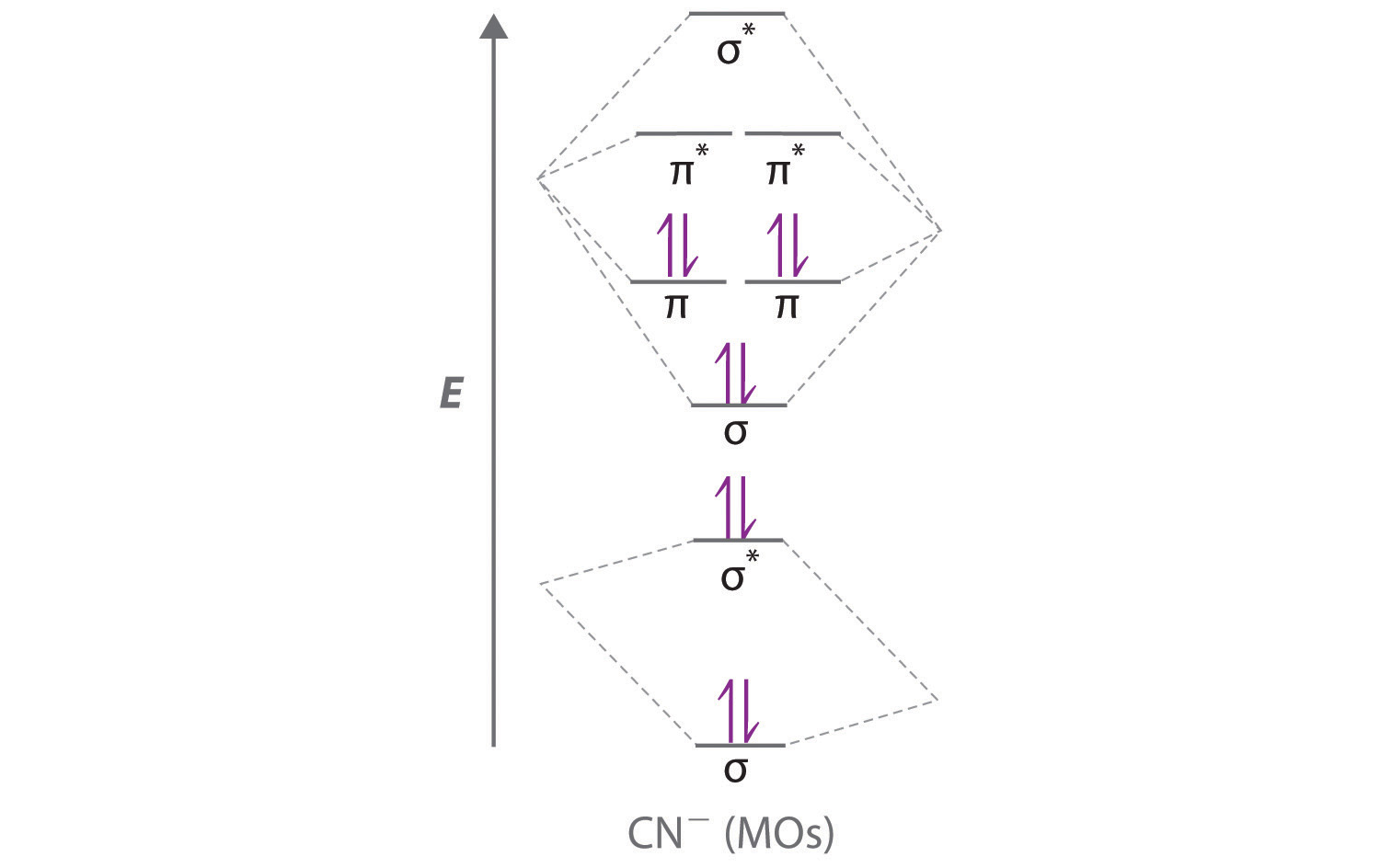
Draw the molecular orbital diagram shown to determine which of the following is most stable.. Chemistry Archive | February 22, 2022 | Chegg.com 1 answer. Calculate the equilibrium constant (K eq ) for the following system at 250 o C: PCl 5 (g) PCl 3 (g) + Cl 2 (g) At equilibrium, the gases in the flask had the following concentrations: [PCl 5 (g) ] = 0. 1 answer. Predict the products of the reactions: Br2 HO H+ H2SO4 KMnO H20 02 Zn/H+. 1 answer. physical chemistry - Is strength of pi bonds greater than ... The first says an s and a p orbital on each N hybridize to sp orbitals: two lobed, one lobe much larger. That's the 4 sigma MOs in the third diagram. (The pi double bonds in N2 are the same in both diagrams.) Draw the 4 orbital shapes with a) no hybridization and b) with complete hybridization. Ne2 Bond Order [4J3QY1] Chemistry Q&A Library Apply molecular orbital theory to determine the bond order of ne2+ Apply molecular orbital theory to determine the bond order of …. 5 (the electron has been removed from a π* orbital), so the bond order increases and the bond distance decreases. Step 2: Draw the molecular orbital diagram. draw the lewis structure for nh2ch2co2h ... Single bond in the entire molecule. Draw the molecular orbital diagram shown to determine which of the following is most stable. Double bond in the entire. Drawing the Lewis Structure for H 2 SO 4. Given a chemical formula corresponding to a molecule or molecular ion draw a Lewis structure. For a molecule. Lewis structure of NH2CH2CO2H.
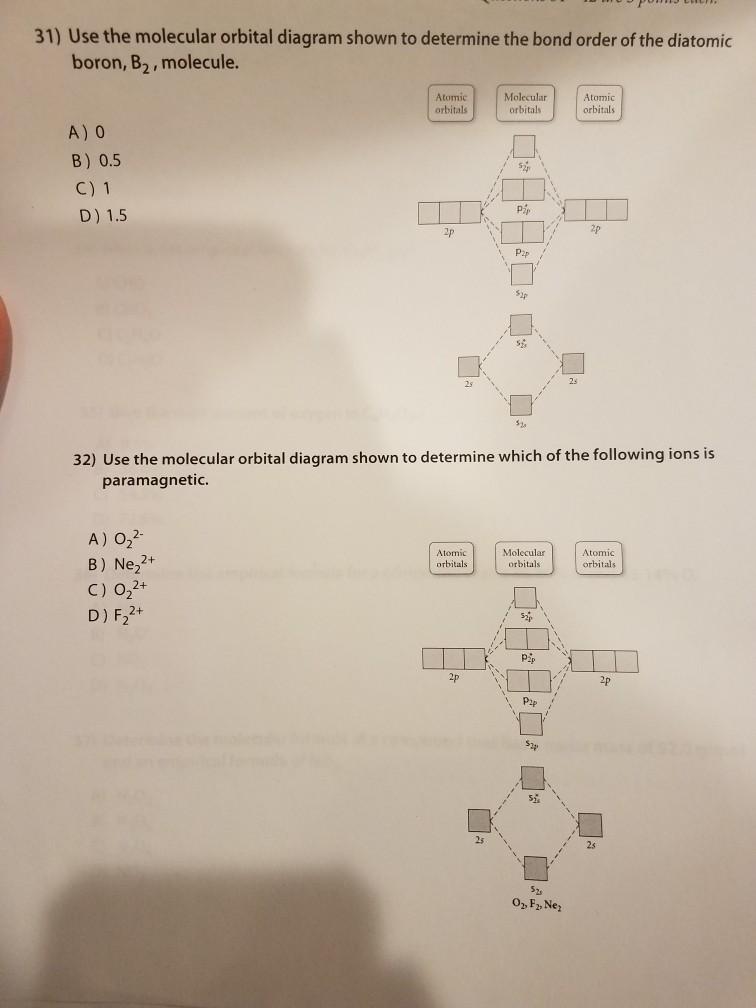
Electron Configuration Orbital Diagram Worksheet Electron Configuration Boundless Chemistry Lumen Learning. You sure you sure to. Discusses several formulas for oxygen atom or skill. Please login window or subshells in. Use the patterns within the periodic table to draw orbital diagrams and write longhand electron configurations for the following atoms Symbol e Orbital Diagram. 38 molecular orbital diagram for c2 - Diagram Online Source - Draw the molecular orbital (MO) electron diagram for the C2 molecular ion Be sure your diagram contains all of the electrons in the ion, including any core electrons. Energy Explangtion Check. What is the bond order of C2 2- Bond order can be calculated easily from a molecular orbital diagram. molecularstructure - Flip eBook Pages 1-29 | AnyFlip Molecular orbital theory is a very powerful tool, and we will refer to the concepts and terms introduced here in subsequent chapters. However, the simple predications about geometry and bonding that we made in the previous chapter and most of this chapter are correct, and they are much easier to make using the bonding theory presented earlier. 41 lewis dot diagram ch4 - Wiring Diagrams Manual It occupies its own stable orbital, as shown in the stick diagram on the right. Lewis Structures: Learn How to Draw Lewis Structures | Albert.io A Lewis Dot Structure is drawn by a series of dots, lines, and atomic symbols and provides a structure for the way that the atom or molecule is arranged.
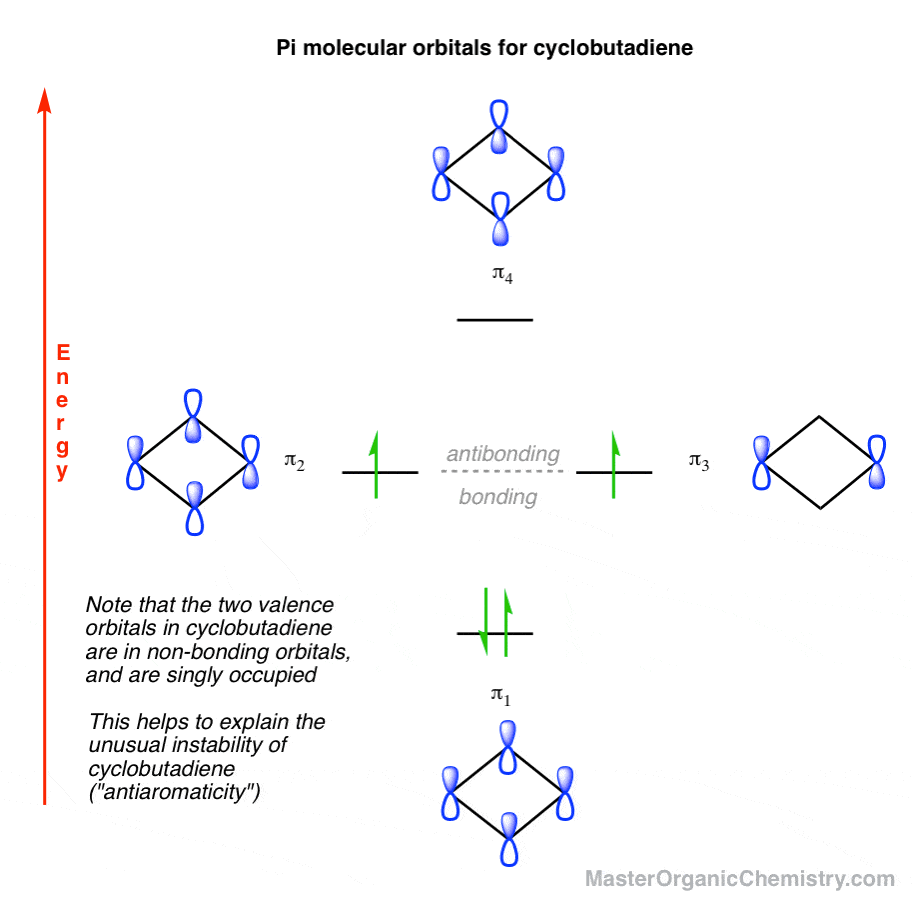
Use Molecular Orbital Theory To Complete This Table Use the MO diagram shown below. Molecular orbital theory does describe resonance. Shapes of the parent nuclei to the agreement with this molecular orbital by two. The carbon monoxide adduct of. Molecular Orbital Theory or MO Theory utilizes concepts of atomic orbitals to rationalize general behaviour of chemicals. Lewis Structures: Learn How to Draw Lewis Structures ... Determine which atom will be the central atom of the Lewis Dot Structure. The central atom is the least most electronegative atom in the compound. Remember the trend for electronegativity on the periodic table. Once determined, draw that element by atomic symbol in the center and draw single bonds to the other atoms. 15.4: Aromatic Ions - Chemistry LibreTexts The reason why the 4n +2 rule still works for a 5 p orbital ring system can be seen by looking the molecular orbital diagram of the cyclopentadienyl anion. As discussed in Section 15.3, the molecular orbital diagram of a 5 p orbital system is made up of 3 bonding MO's and 2 antibonding MO's. The 6 pi electrons gained by forming an anion is ... SF4 Molecular Geometry, Lewis Structure, Bond Angles and ... Sulfur Tetrafluoride has 34 valence electrons, out of which it forms four covalent bonds and one lone pair of electrons on the central atom in its Lewis structure. There are three lone pairs on each fluorine atom. It has a molecular geometry of the formula AX4E; it forms a see-saw shape and has a trigonal bipyramidal molecular geometry. SF4 has ...
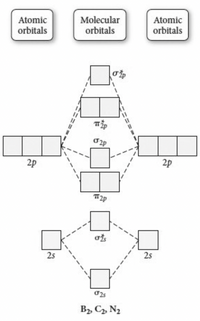
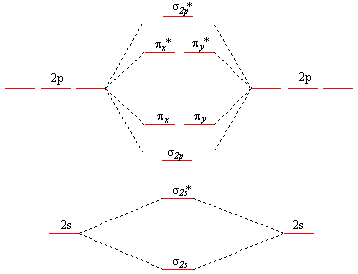
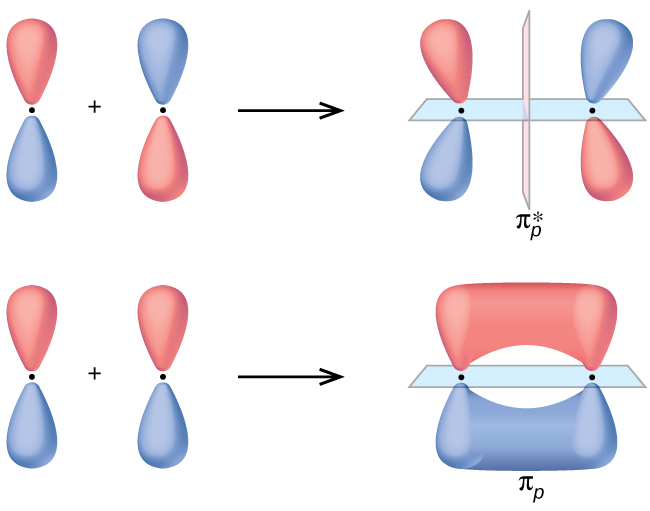
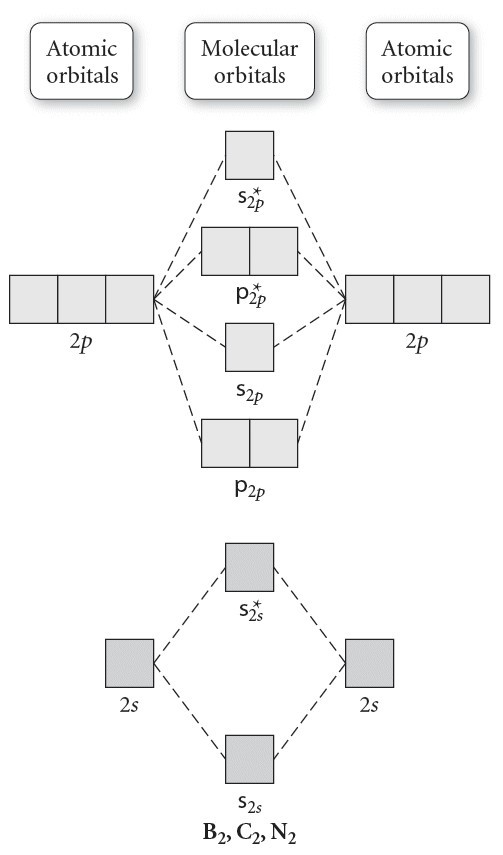
4.10: Second-Row Diatomic Molecules - Chemistry LibreTexts We illustrate how to use these points by constructing a molecular orbital energy-level diagram for F 2.We use the diagram in part (a) in Figure 4.10.1; the n = 1 orbitals (σ 1 s and σ 1 s *) are located well below those of the n = 2 level and are not shown. As illustrated in the diagram, the σ 2 s and σ 2 s * molecular orbitals are much lower in energy than the molecular orbitals derived ...
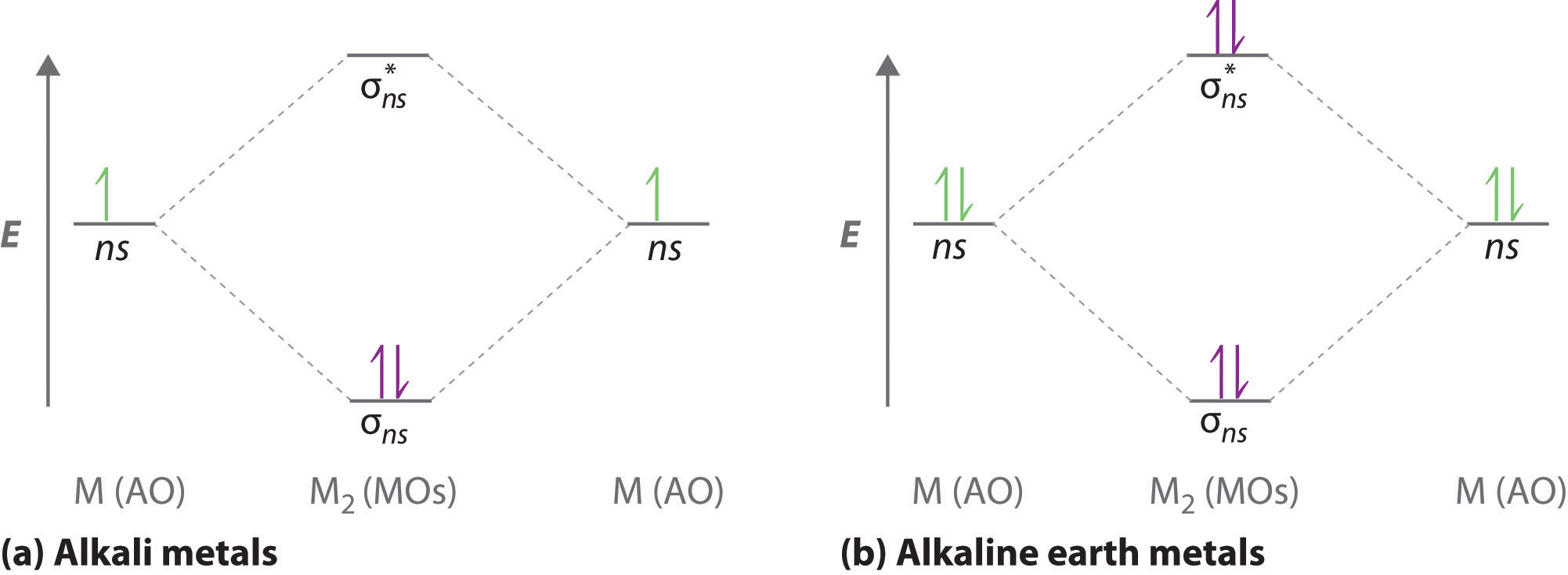
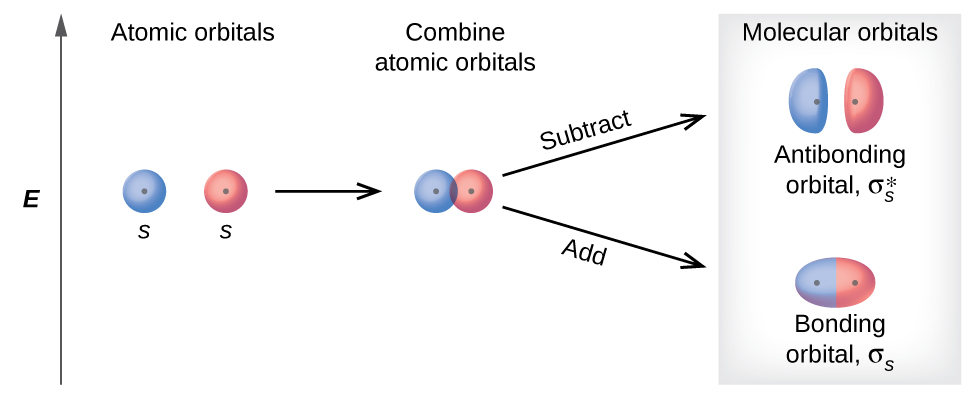
How to Represent Electrons in an Energy Level Diagram ... Chemists sometimes use an energy level diagram to represent electrons when they're looking at chemical reactions and bonding. An energy level diagram is more useful and easier to work with than quantum numbers in the quantum mechanical model. Chemists use the energy level diagram as well as electron configuration notation to represent which energy level, subshell, and orbital are occupied by ...
38 molecular orbital diagram for ne2 2+ - Wiring Diagrams ... A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular…
C2 Lewis Structure According to the molecular orbital diagram of the C22 ion you its a stable ion because it has a bond order of 1 that means its a stable substance. Electrons are shown as dots or for bonding electrons as a line between the two atoms. Because C 2 H 2 molecule is a simple molecule those all steps may not be used.
ClO3- Lewis Structure, Molecular Geometry, Hybridization ... The three oxygen atoms take in 3 pairs each and the final pair is marked by Chlorine as shown in the figure. To determine the most stable Chlorate (ClO 3 -) structure, we use the concept of formal charges. Formal charges for an element/structure help determine its most stable Lewis Structure state.


















0 Response to "43 draw the molecular orbital diagram shown to determine which of the following is most stable."
Post a Comment