42 solidus line phase diagram
L - Engineering Archives The solvus is represented by a line on a phase diagram that separates a solid phase from a solid1 + solid2 phase, where solid1 and solid2 are different microstructures. The eutectic is represented by the horizontal line in a eutectic binary phase diagram, connecting the intersections of the solidus and solvus lines from both sides. chapter 9: phase diagrams Flashcards | Quizlet On a binary phase diagram, the line or boundary separating liquid- and liquid + solid-phase regions. For an alloy, the liquidus temperature is the temperature at which a solid phase first forms under conditions of equilibrium cooling.
Features of phase diagram | bartleby Solidus: The solidus line is a line on a phase diagram, below which a substance acts as solid. It is the locus of temperature below which it is only characterized by solid. The solid is characterized by large intermolecular forces. Liquidus: It is a line in the phase diagram above which is characterized by only liquid. Intermolecular forces are ...
Solidus line phase diagram
Solved A transformation line on a phase diagram indicating ... A transformation line on a phase diagram indicating the temperatures at which solidification commences under slow cooling is called a line. OI. solvus o Il liquidus O III Solidus O IV tie line. The highest temperature at which only a solid exists at any composition is referred to as the 01 solidus O 11. liquidus O II. 34. Binary Phase Diagrams: Complete Solubility MIT OpenCourseWare is a web-based publication of virtually all MIT course content. OCW is open and available to the world and is a permanent MIT activity Phase diagram - Wikipedia A typical phase diagram. The solid green line shows the behaviour of the melting point for most substances; the dotted green line shows the anomalous behavior of water. The red lines show the sublimation temperature and the blue line the boiling point, showing how they vary with pressure.
Solidus line phase diagram. Solidus (chemistry) - Wikipedia In chemistry, materials science, and physics, the solidus is the locus of temperatures (a curve on a phase diagram) below which a given substance is completely solid (crystallized). Supercritical fluid - Energy Education In the phase diagram of water (figure 1), as the temperature and pressure increases, water starts to travel across the solidus line, and reaches the triple point. Triple point denotes a temperature and pressure when all the three phases are present in the water. CHAPTER 9 PHASE DIAGRAMS PROBLEM SOLUTIONS 9.34 Consider the hypothetical eutectic phase diagram for metals A and B, which is similar to that for the lead-tin system, Figure 9.8. Assume that (1) α and β phases exist at the A and B extremities of the phase diagram, respectively; (2) the eutectic composition is 47 wt% B-53 wt% A; and (3) the composition of the β phase at Line the line on a phase diagram representing the line- The line on a phase diagram representing the limit of solid solubility as a function of temperature. Solidus line- The line on a phase diagram at which solidification is complete upon equilibrium cooling, or at which melting begins upon heating. Liquidus line-The line indicating above which melting of a solid is complete upon heating or ...
Study on the Solidus Line in Sn-Rich Region of Sn-In Phase ... It is shown that the position of solidus line can be verified and the dotted line can be replaced by a solid line in Sn-rich region of Sn-In phase diagram. Table 1 The average compositions of solid phase grains in Sn70In30, Sn75In25, Sn80In20, and Sn85In15 alloys after annealing treatment Full size table Fig. 3 Above the following line, liquid phase exist ... - Sarthaks Above the following line, liquid phase exist for all compositions in a phase diagram. A. Tie-line B. Solvus C. Solidus D. Liquidus Liquidus Line - ScienceDirect.com For the simple diagram of Figure 2.18 (a) horizontal sections from room temperature up to the solidus surface show no variation, merely solid phase. At temperature T, the horizontal section cuts through the (solid+liquid) phase field and looks like Figure 2.18 (b); alloys can be liquid, solid or (solid+liquid) depending on the composition. Eutectic system - Wikipedia Conversely, when a well-mixed, eutectic alloy melts, it does so at a single, sharp temperature. The various phase transformations that occur during the solidification of a particular alloy composition can be understood by drawing a vertical line from the liquid phase to the solid phase on the phase diagram for that alloy. Some uses include:
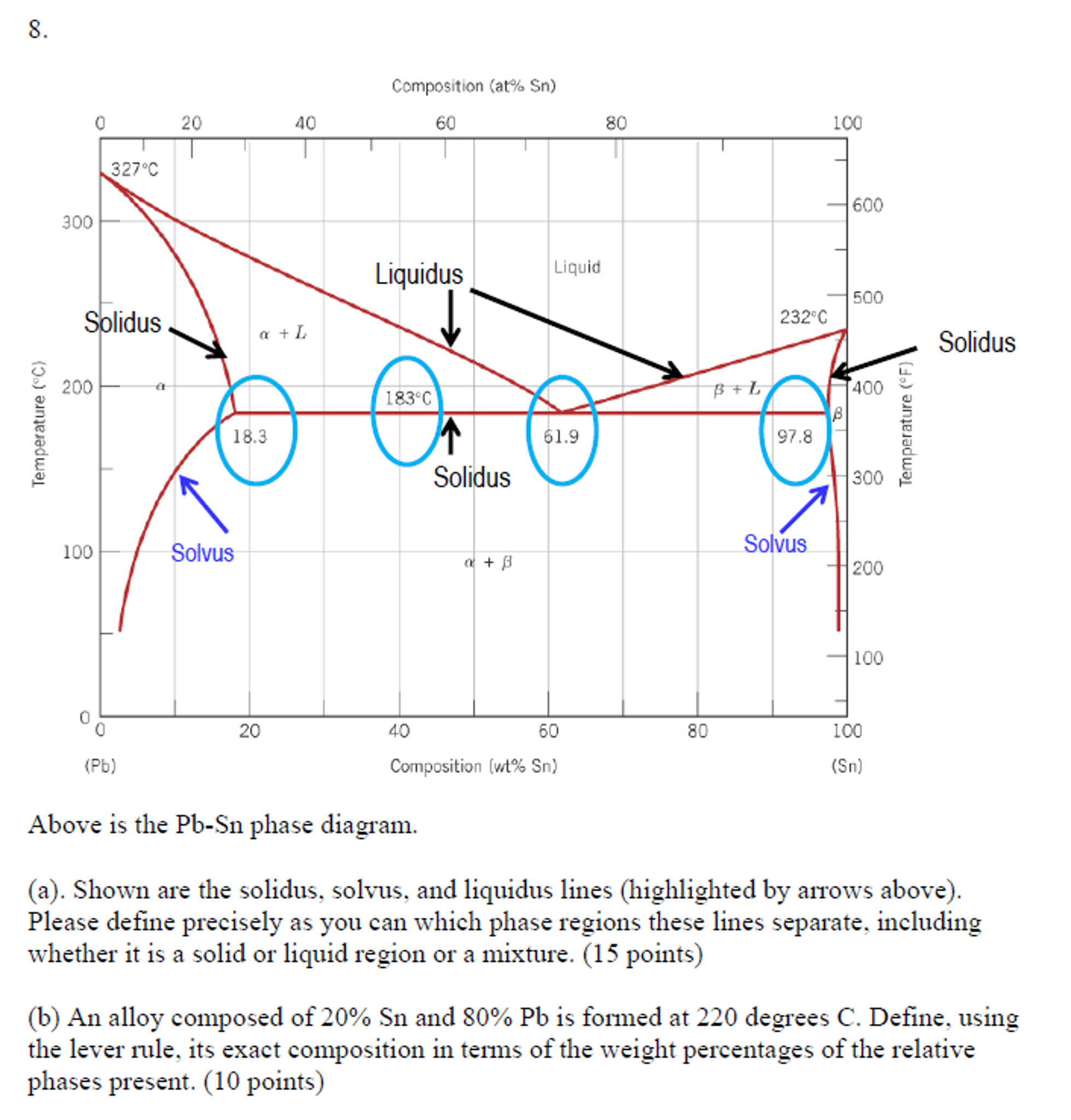
materials - Princeton University The equilibrium "phase diagram" opposite shows the dependence of the alloys melting point on its composition, and the existence of the single solid phase below the solidus line indicates the complete solid state miscibility of the two elements. The Solidus Line of the Cu-Bi Phase Diagram | SpringerLink It is shown that the solidus line is a retrograde curve without a monotectic transition. The solid and liquid phases are successfully described with simple thermodynamic models. The experimentally measured maximum solubility of 0.0207 at. % Bi at 975 °C correlates well with that from the model (0.0193 at. % Bi at 968 °C). Question. 1. In the Pb-Sn phase diagram shown below ... Point out the liquidus, solidus and solvus phase boundary lines (10 points) b. Schematically draw the microstructures at points "A", "B" and "C" for an alloy of composition "Co" (see dotted line in the phase diagram), (10 points) Pb-Sn system T (° C) A B 400 300 2001 TE 100 L 10 L + a a + ß I 20 Co 30 C, This question hasn't been solved yet PDF The Solidus Line of the Cu-Bi Phase Diagram Journal of Phase Equilibria Vol. 18 No. 2 1997 The Solidus Line of the Cu-Bi Phase Diagram L.-S. Chang, B.B. Straumal*, E. Rabkin, W. Gust, and F. Sommer Max-Planck-Institut für Metallforschung Institut für Metallkunde der Universität Seestrasse 75, D-70174 Stuttgart, Germany (Submitted 18 September 1996; in revised form 8 November 1996)
What is tie line in phase diagram? - FindAnyAnswer.com Solidus line. The temperature occurring as a function of the composition of an alloy at which the cooling melt of this alloy completely solidifies is known as its solidus line (analogous to the liquidus line) . Between these two lines, a mixture of the metals is present in liquid and solid phases. What is eutectic phase diagram?
COPPER - NICKLE PHASE DIAGRAMS (LECTURE 9) Flashcards ... The solidus line changes indicating solidifying and melting will happen at cooler temperatures, the faster the cooling the the greater the difference seen in the phase diagram How can we use heat to help non-equilibrium solidification
PDF Chapter Outline: Phase Diagrams Isomorphous system -complete solid solubility of the two components (both in the liquid and solid phases). Binary Isomorphous Systems (I) Three phase region can be identified on the phase diagram: Liquid (L) , solid + liquid (α +L), solid (α ) Liquidus line separates liquid from liquid + solid Solidusline separates solid from liquid + solid α+ L α
PDF Isomorphous Phase Diagrams in Mathcad The simplest phase diagram is one in which the two solids can be mixed in any proportions as a single solid phase. These are called isomorphous diagrams. A common example of such a diagram is the copper/nickel system. This article describes a Mathcad worksheet that can create and analyze an isomorphous phase diagram.
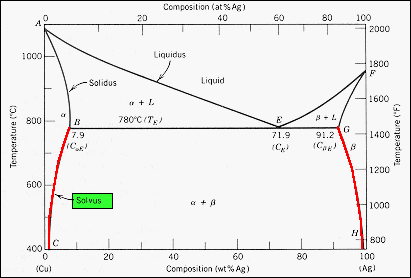
nglos324 - solidus - Princeton The solidus lines on a phase diagram represent the locus of all points that represent the completion of the solidification of a material of given composition as it is cooled in equilibrium from the liquid phase. For the copper-silver binary phase diagram shown two such lines exist, one for the
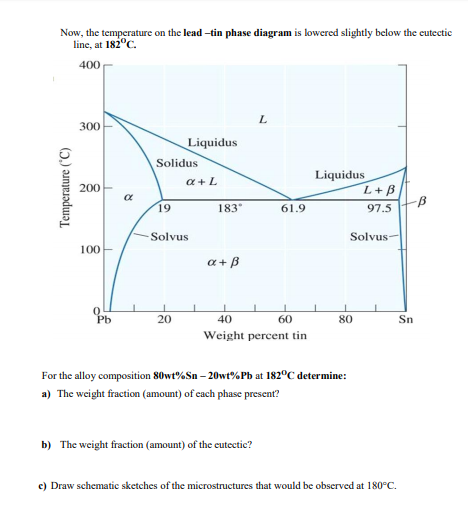
Chapter 9: Phase Diagrams - Florida International University in 2-phase region: 1. Draw the tie line. 2. Note where the tie line intersects the liquidus and solidus lines (i.e. where the tie line crosses the phase boundaries). 3. Read off the composition at the boundaries: Liquid is composed of CL amount of Ni (31.5 wt% Ni). Solid is composed of Cαααα amount of Ni (42.5 wt% Ni).
Phase Diagram: Meaning and Types - Engineering Notes India This phase diagram consists of two points, two lines and three areas. The two points of the two pure metals A & B. The upper line, obtained by connecting the points showing the beginning of solidification is called liquidius line, and the lower line, determined by connecting the points showing the end of solidification is called the solidus line.
250+ TOP MCQs on Binary Phase Diagrams and Answers Clarification: Isomorphous phase diagram is the phase diagram in which the two components are soluble in each other over an entire range of composition in both solid and liquid phases. 4. What is the XY line in the given phase diagram? a) Lever line b) Tie line c) Solidus line d) Liquidus line Answer: b
Solidus Line - ScienceDirect.com For the simple diagram of Figure 2.18 (a) horizontal sections from room temperature up to the solidus surface show no variation, merely solid phase. At temperature T, the horizontal section cuts through the (solid+liquid) phase field and looks like Figure 2.18 (b); alloys can be liquid, solid or (solid+liquid) depending on the composition.
Solid Solution Phase Diagram - James Madison University (numbers on phase diagram correspond with numbers below) 1. As an example, begin with a hot melt of 30% anorthite. 2. Cool melt to liquidus line. First crystal begins to form at about 1380 o. 3. To determine the composition of the first crystal move horizontally across the diagram to the solidus line. The solidus always indicates crystal ...
(PDF) The Solidus Line of the Cu-Bi Phase Diagram The Solidus Line of the Cu -Bi Phase Diagram L.-S. Chang, B.B. Straumal*, E. Rabkin, W. Gust, and F. Sommer Max-Planck- Institut für Metallforschung Institut für Metallkunde der Universität...
Liquidus vs Solidus - What's the difference? - WikiDiff As nouns the difference between liquidus and solidus is that liquidus is (chemistry|physics) a line, in a phase diagram, above which a given substance is a stable liquid and below which solid and liquid are in equilibrium while solidus is the line between the numerator and the denominator of a fraction.
PDF Chapter 9 Phase Diagrams - KSU Three phase region can be identified on the phase diagram: Liquid (L) , solid + liquid (α +L), solid (α ) Liquidus line separates liquid from liquid + solid Solidus line separates solid from liquid + solid Binary Isomorphous Systems (II)
Phase diagram - Wikipedia A typical phase diagram. The solid green line shows the behaviour of the melting point for most substances; the dotted green line shows the anomalous behavior of water. The red lines show the sublimation temperature and the blue line the boiling point, showing how they vary with pressure.
34. Binary Phase Diagrams: Complete Solubility MIT OpenCourseWare is a web-based publication of virtually all MIT course content. OCW is open and available to the world and is a permanent MIT activity
Solved A transformation line on a phase diagram indicating ... A transformation line on a phase diagram indicating the temperatures at which solidification commences under slow cooling is called a line. OI. solvus o Il liquidus O III Solidus O IV tie line. The highest temperature at which only a solid exists at any composition is referred to as the 01 solidus O 11. liquidus O II.




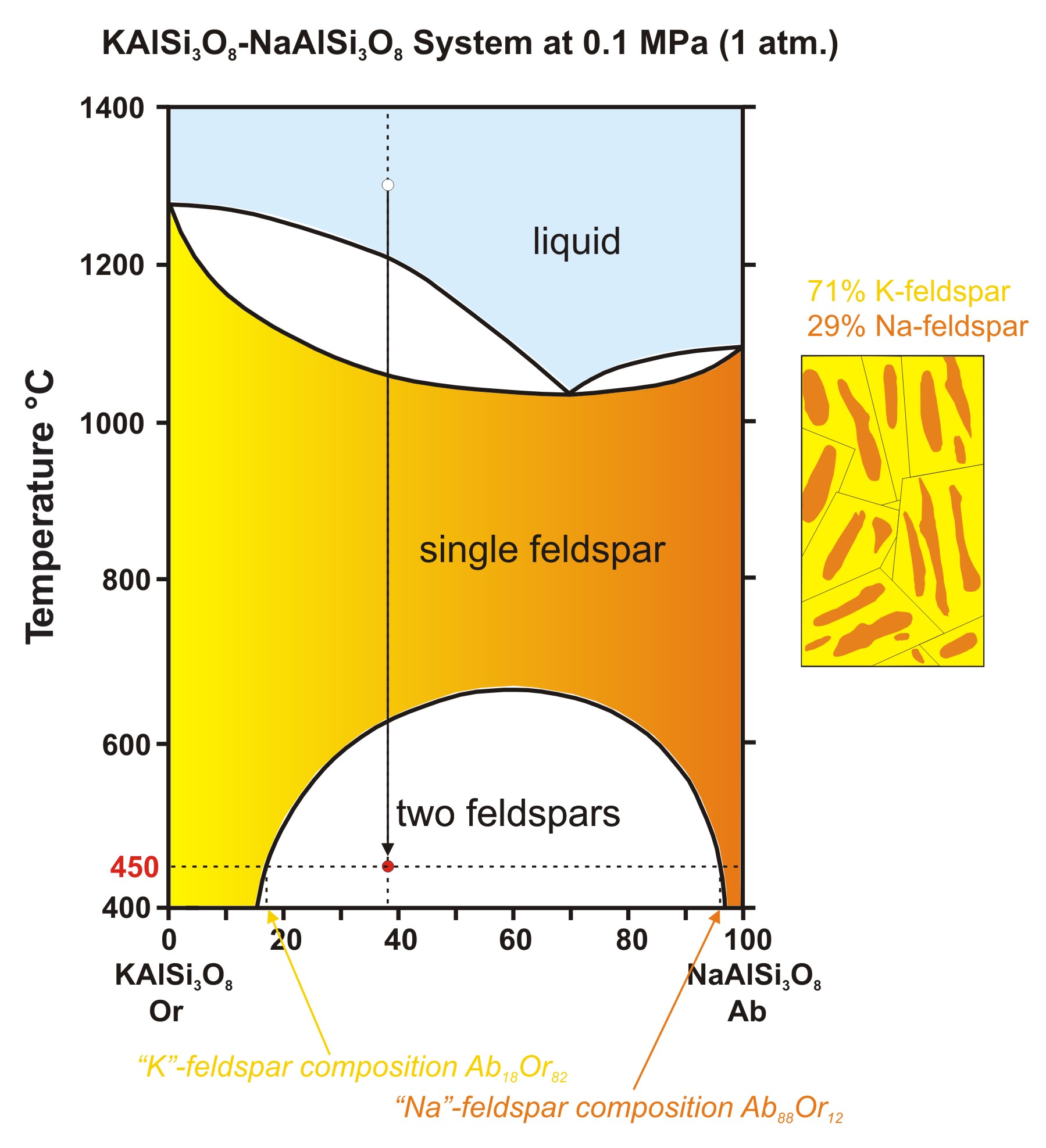

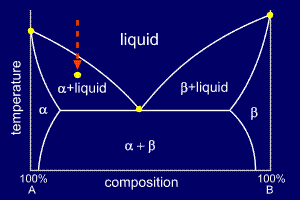


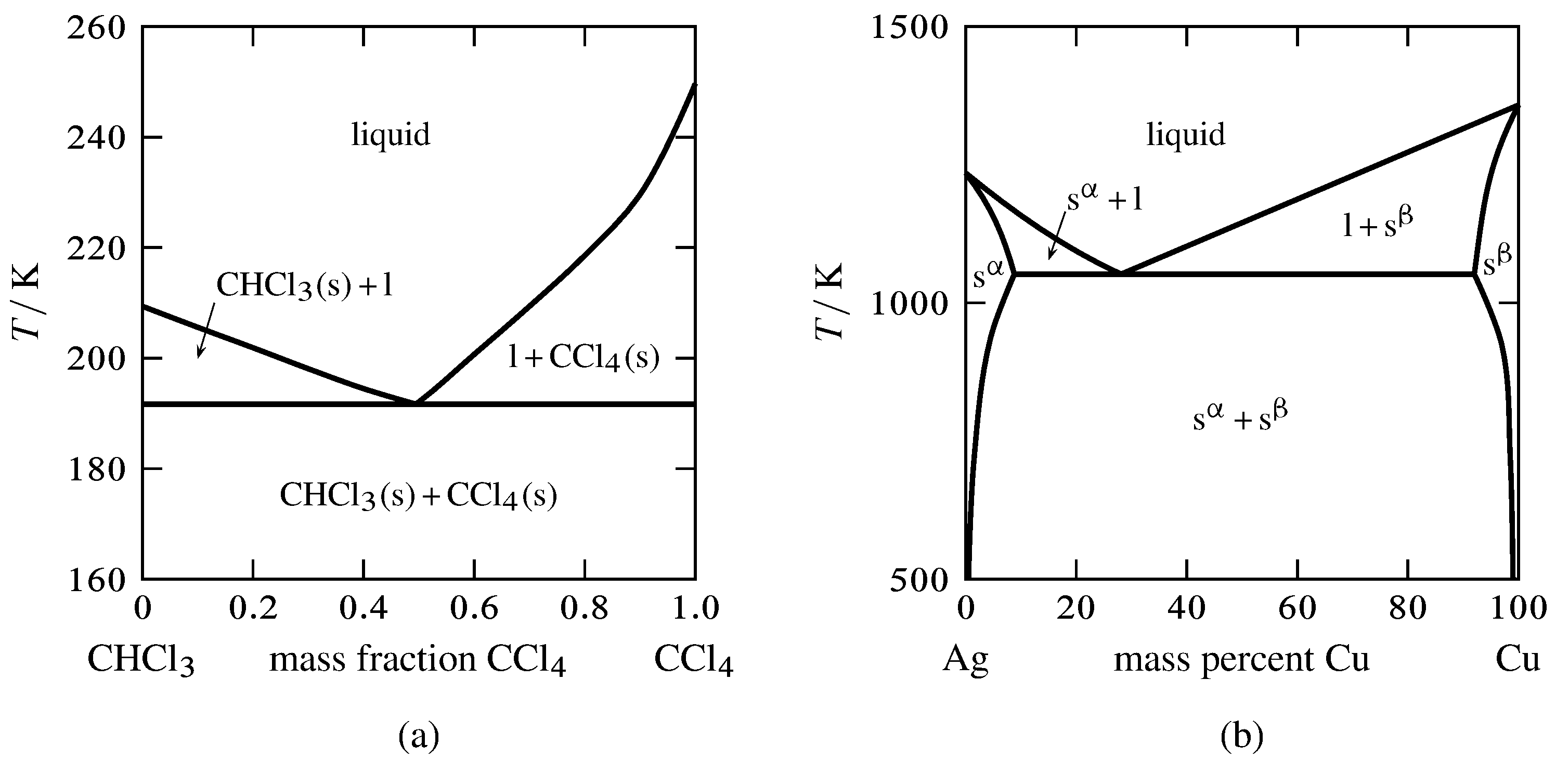

![Phase transformations and phase diagrams [SubsTech]](https://www.substech.com/dokuwiki/lib/exe/fetch.php?w=&h=&cache=cache&media=peritectic.png)

![Iron-Carbon Phase Diagram Explained [with Graphs]](https://fractory.com/wp-content/uploads/2020/03/Iron-carbon-phase-diagram-explained.jpg.webp)

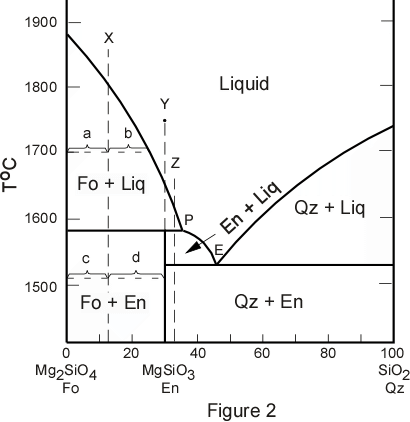




0 Response to "42 solidus line phase diagram"
Post a Comment