45 labeled reaction coordinate diagram
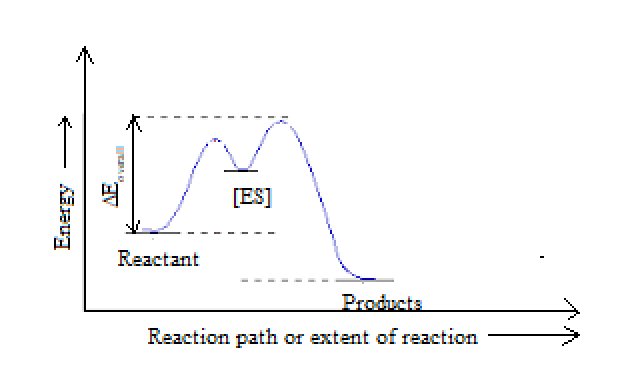
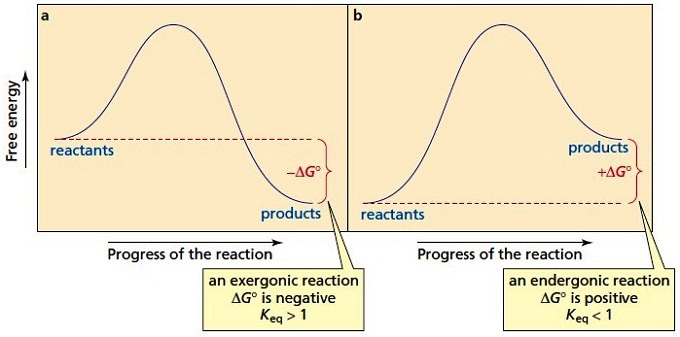
Answered: a) Draw the reaction coordinate diagram… | bartleby a) Draw the reaction coordinate diagram (reaction progress vs. energy) that is consistent with this mechanism. Assume the reaction is exothermic. Label where is ES appears in the diagram. The Michaelis-Menten equation gives the rate law for this mechanism: Rate = k2 [E]o [S]/ (Ka + [S]) b) With an understanding of this rate law, identify which ... Endergonic Reaction Diagram - Wiring Diagrams Reaction coordinate diagrams of exergonic and endergonic reactions. Exergonic and endergonic reactions are characterized by changes in. Exergonic and endergonic qualifications only apply for Gibbs' free energy. Enthalpy applies to potential energy diagrams. Endergonic just. The activation energy shown in the diagram below is for the forward ...
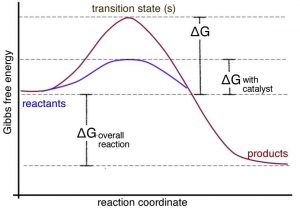
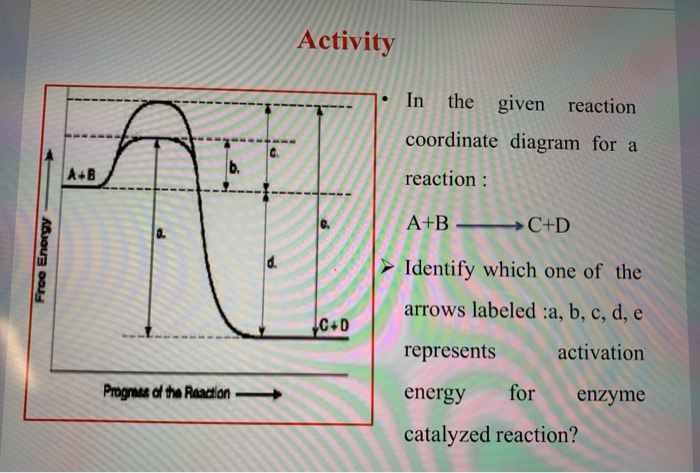
Energy Diagram Catalyzed Vs Uncatalyzed Reaction However, the detailed mechanics of catalysis is complex. c. Consider the catalyzed reaction. Reactants Products + Energy. Draw a reaction coordinate diagram for this reaction as above but add the activation energy, E a, for the catalyzed reaction on the appropriate curve in this diagram and label it.
Labeled reaction coordinate diagram
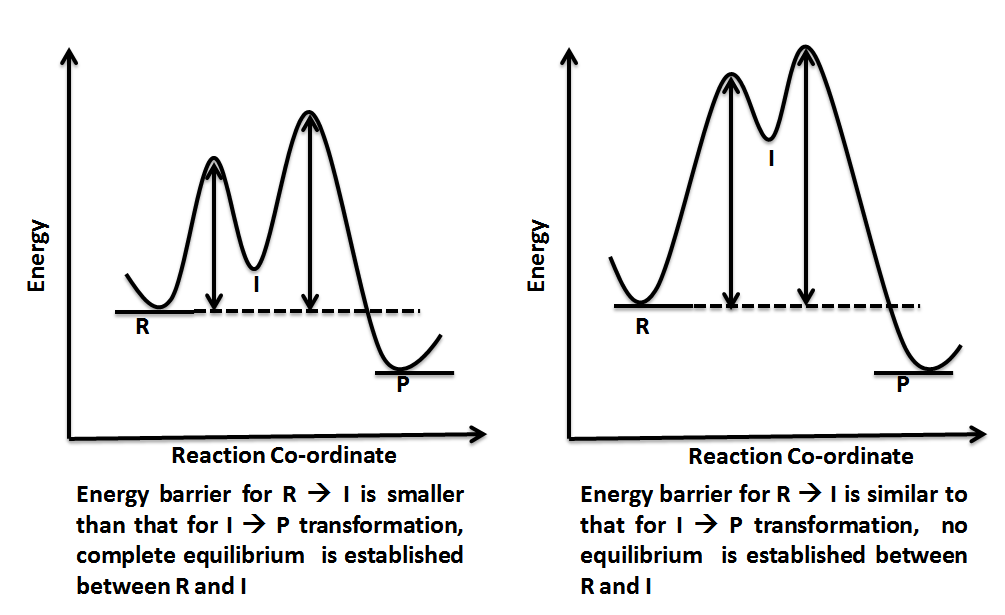
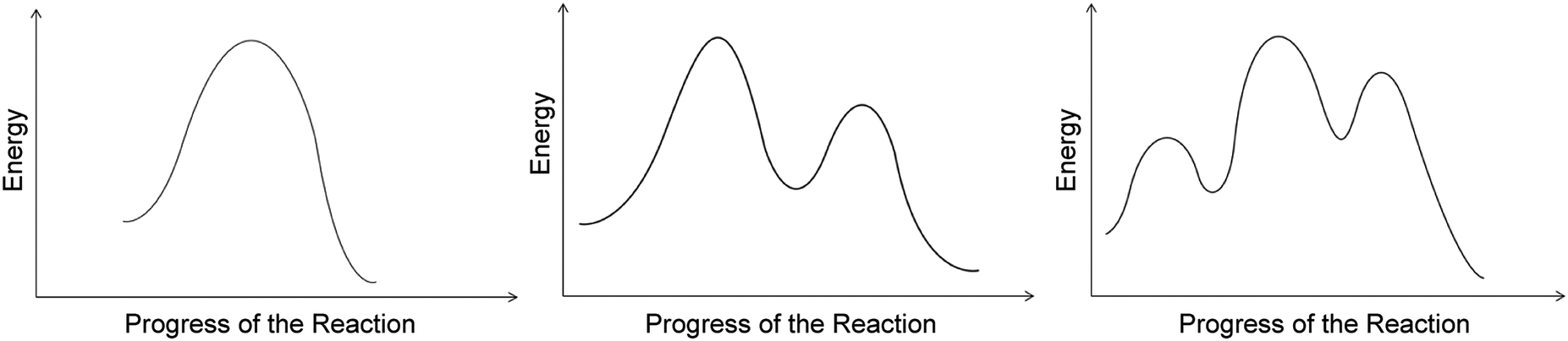
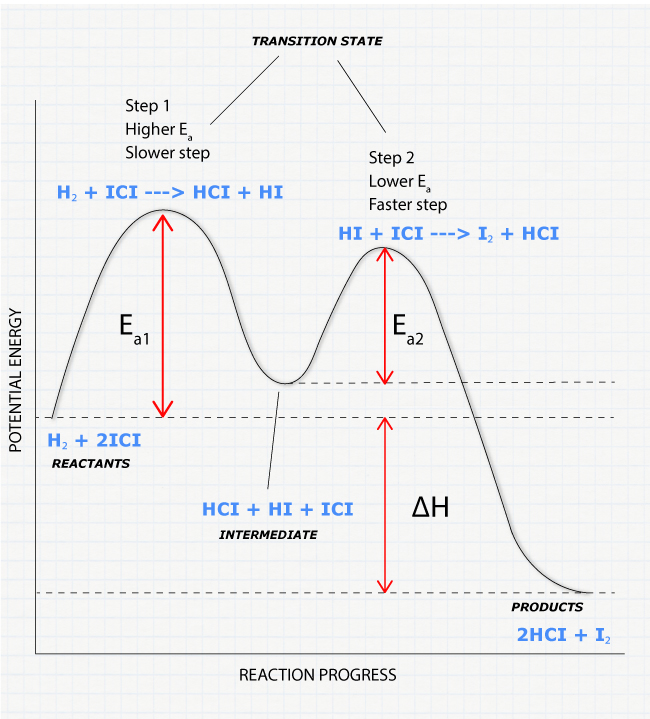
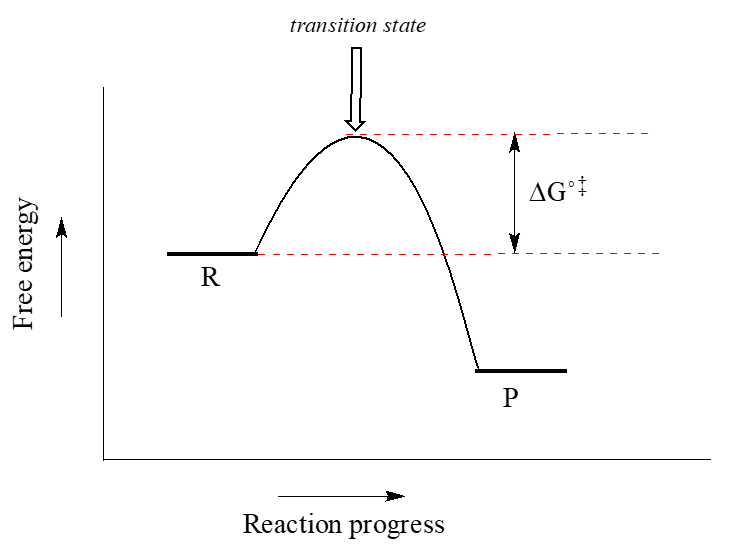
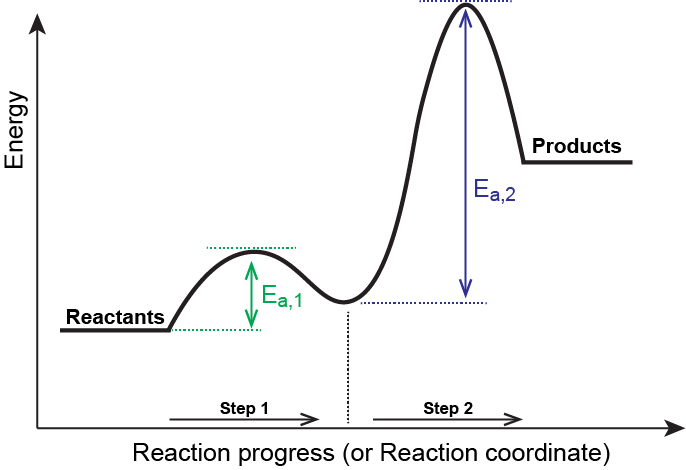
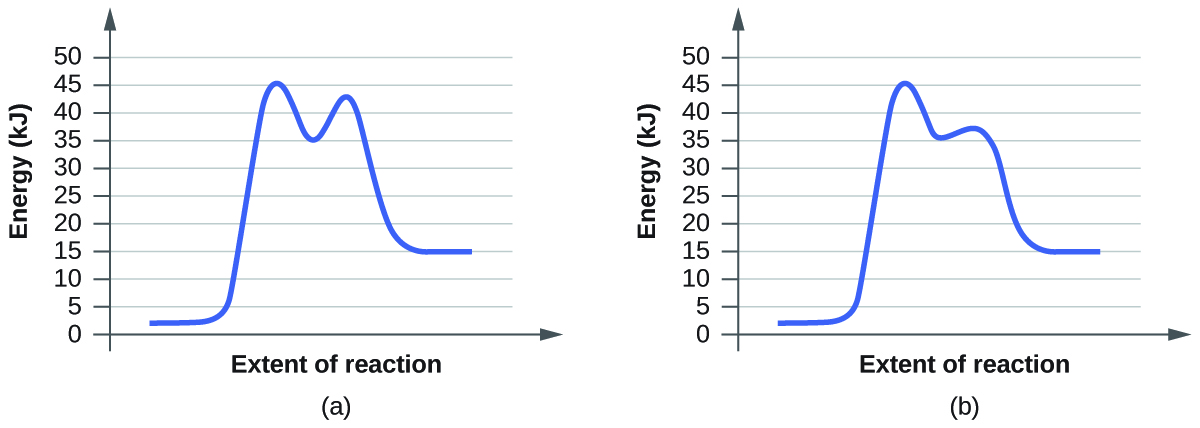
Energy Diagrams, Transition States, and Reactive ... Energy diagrams are used to represent the change in energy for the molecules involved in a chemical reaction. The free energy is measured along the y-axis, and the reaction coordinate is plotted on the x-axis.. The reaction coordinate indicates the progress of the conversion of reactants to products. 6. Reaction Coordinate Diagram - VIZISCIENCE ... Reaction Coordinate Diagram. Given the following reaction, sketch a reaction coordinate graph. The reaction involves two steps, step 1 is the slowest step and ... Arrhenius Theory and Reaction Coordinates The reaction above has three steps (three barriers) and two intermediates. On the far left of the diagram are the reactant species and on the far right are the product species. Transition State The transition state is the high energy point between two minima along the reaction coordinate. Each step in a mechanism will have a transition state.
Labeled reaction coordinate diagram. Energy Diagram — Overview & Parts - Expii 1) Label the axes, the x-axis is labeled with reaction coordinate, and the y-axis is labeled with energy · 2) Draw a line at the beginning of the graph for ... 6.6: Reaction Coordinate Diagrams - Chemistry LibreTexts In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical one-step reaction might look like this: Description of the Reaction Coordinate - YouTube Explains the energy versus reaction coordinate diagram. Relates the activation energies for the forward and reverse reactions of an exothermic reaction to th... PDF Energy/Reaction Coordinate Diagrams 1! Energy/Reaction Coordinate! Diagrams! Thermodynamics, Kinetics ! Dr. Ron Rusay" A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction
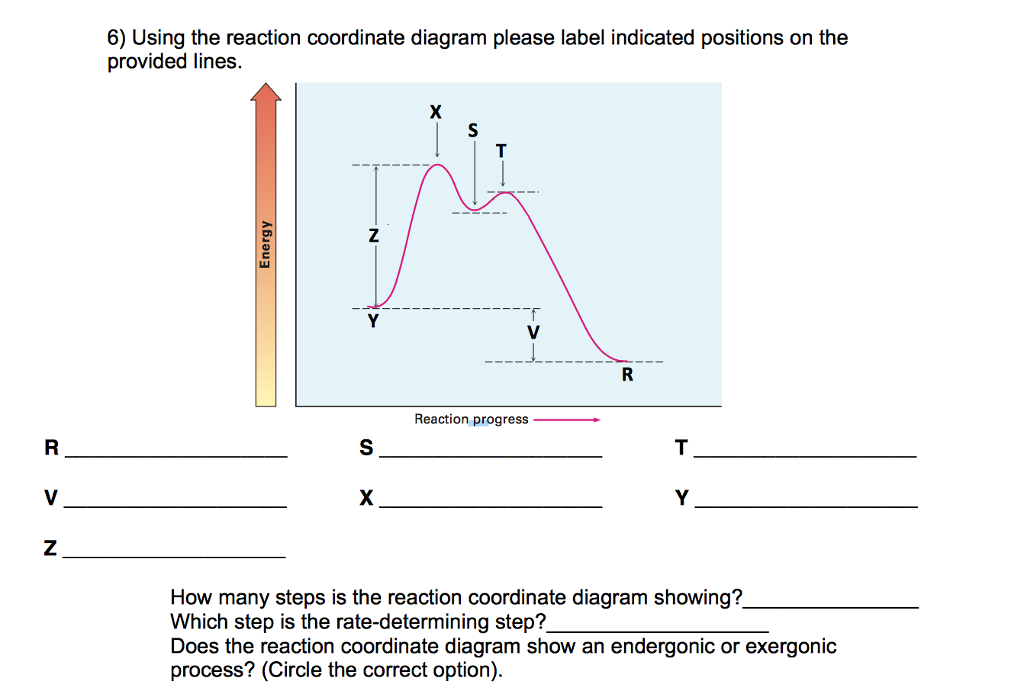
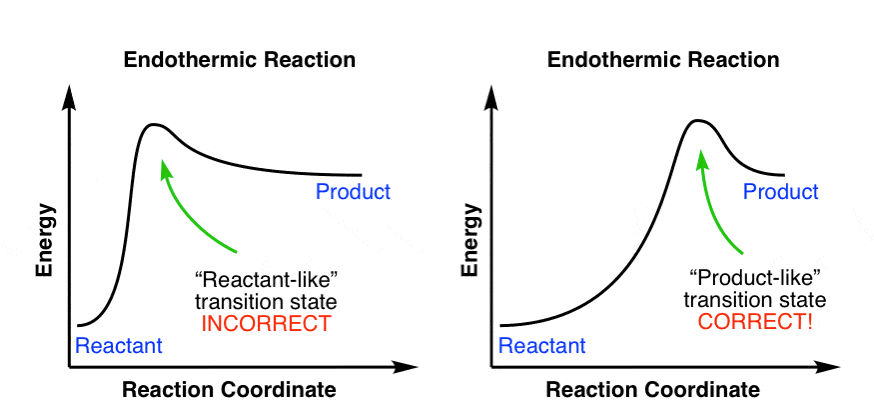
Endothermic Reaction Coordinate Diagram - schematron.org The fully filled in reaction coordinate diagram is displayed below. This reaction is also exothermic because the energy of the products is lower than that of the. In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other.Start studying CHEMISTRY 2. Solved Label the following reaction coordinate diagram ... Label the following reaction coordinate diagram. Energy Reactant (s) Transition State Product (s) Activation Energy (forward) Transition State Activation Energy (forward) Energy Enthalpy of Enthalpy of Reaction Product (s) Reaction AHrxn Reactant (s) Reaction Coordinate Reaction Coordinate Reset Zoom Reaction Coordinate Diagram practice problems Quiz - Quizizz What does arrow #1 represent in this reaction coordinate diagram represent? What does arrow #3 represent in this reaction coordinate diagram represent? What is the change in enthalpy (ΔH rxn) of the reaction in the picture? What is the potential energy of the products (PE products) in the graph in the picture? Label The Following Reaction Coordinate Diagram ... Label the following reaction coordinate diagram enthalpy of reaction activation energy (forward) reactant(s) transition state = 0 . Start by drawing and labeling the reaction coordinate diagram. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams.
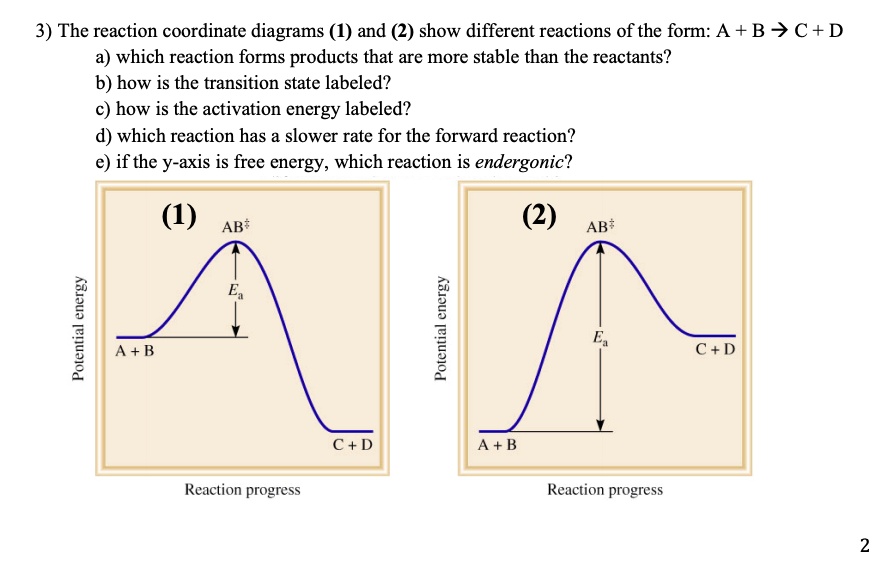
College Chemistry : Reaction Coordinate Diagrams ... Explanation: Start by drawing and labeling the reaction coordinate diagram. Since we know that is negative, the products must be lower in energy than the reactants. Now, the question wants you to find the activation energy if we were to reverse the reaction. In other words, we want to start at the products and end up with the reactants. Reaction coordinate - Wikipedia Reaction coordinate. Diagram of a catalytic reaction, showing the energy niveau as a function of the reaction coordinate. For a catalyzed reaction, the activation energy is lower. In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter ... Labeling Parts of a Reaction Coordinate Diagram - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... chem (kinetics pt 2) Flashcards | Quizlet Label the following reaction coordinate diagram by matching between letters and numbers: (diagram in kinetics pt 2 folder in energy diagram folder on desktop) 1- J ... -Label the multi-step reaction energy diagram below using the letters corresponding to the labels on the left. There are more labels than needed; each label can be used only once.
Answered: Spring 2020 rev. 10/22/2018 CHEM S1 ... - bartleby 8. A lit fuse was required to initiate the reaction in the flare. Use this information, along with your previous results to correctly label the reaction coordinate diagram shown below. Use these symbols to label each item in your diagram: reactants (R), products (P), reaction enthalpy (AH), and activation energy (Ea).
Reaction Coordinates in Potential Energy Diagrams ... The reaction coordinate is the dihedral angle between groups on the two atoms, which can be easily observed in a Newman projection. Potential energy surfaces for bond rotation are commonly used for conformational analysis of molecules like ethane and butane. More complicated systems
5.3. Reaction coordinate diagrams | Organic Chemistry 1 You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical ...
Label The Energy Diagram (7 Bins) And Indicate Which ... Ⓕ How To Make Hardware Organizer Labels (ep64) Graph 2. 1. Draw a potential energy diagram for an endothermic reaction. 2. Label your drawing with the following letters: A=PE of products. D=Energy of. The y-axis of the Maxwell-Boltzmann graph can be thought of as giving the number of molecules per unit speed.
Reaction Coordinate Diagrams The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction (heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram.
Anatomy of a simple reaction: the hydration of an alkene ... The diagram below shows us the IRC, or intrinsic reaction coordinate for the process (for definitions, see here), the reaction proceeding from left to right as shown in the scheme above, with a (free energy) barrier of 14.4 kcal/mol and exothermic by a similar amount (wB97XD/6-311G(d,p) with a continuum solvation correction for water). One first notices that it is not the smooth bell-shaped ...
SOLVED:Draw and label a reaction coordinate diagram for an ... Draw and label a reaction coordinate diagram for an uncatalyzed reaction, S --> P, and the same reaction catalyzed by an enzyme, E. Susan H. Hamline University. Answer. Given the preceding mechanism for an enzyme-catalyzed reaction, sketch a potential energy profile for the reaction and label the appropriate parts of your graph with $\mathrm{E ...
Solved Draw and label a reaction coordinate diagram for an ... Draw and label a reaction coordinate diagram for an uncatalyzed reaction (S to P) and a reaction catalyzed by an enzyme on the same plot. On your diagram, show the overall exergonic free energy change for each reaction, the transition states, and the activation energies for both the uncatalyzed and catalyzed reactions. Expert Answer
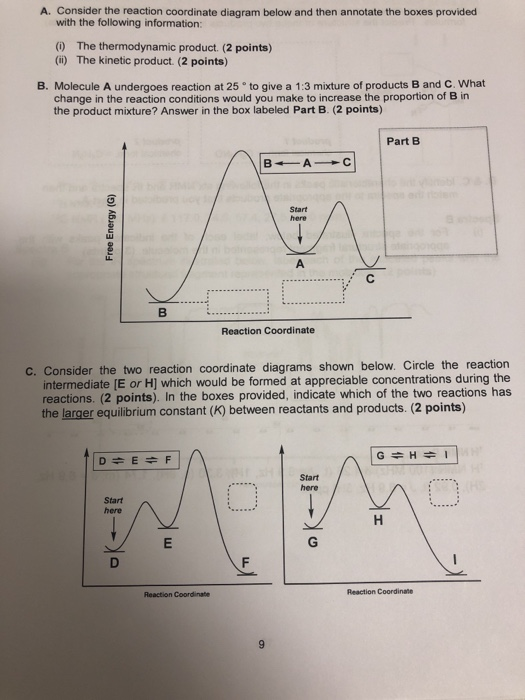
CHEM 233 Exam II Flashcards | Quizlet You know that A can undergo two reactions as shown in the below reaction coordinate diagram with one reaction pathway labeled in red and one reaction pathway labeled in blue. The red path leads to Product B, while the blue path leads to Product C. Assuming both reaction pathways occur simultaneously in competition with each other, what is the ...
OneClass: Labelthe following reaction coordinate diagram ... Labelthe following reaction coordinate diagram by matching betweenletters and numbers: Answer +20. Watch. 1. answer. 0. watching. 90. views. For unlimited access to Homework Help, a Homework+ subscription is required. Jean Keeling Lv2. 10 Aug 2019. Unlock all answers. Get 1 free homework help answer. Unlock ...
How can I draw activation energy in a diagram? | Socratic You follow a series of steps. Explanation: 1. Draw and label a pair of axes. Label the vertical axis "Potential Energy" and the horizontal axis "Reaction Coordinate". 2. Draw and label two short horizontal lines to mark the energies of the reactants and products. 3. Draw the energy level diagram.
Arrhenius Theory and Reaction Coordinates The reaction above has three steps (three barriers) and two intermediates. On the far left of the diagram are the reactant species and on the far right are the product species. Transition State The transition state is the high energy point between two minima along the reaction coordinate. Each step in a mechanism will have a transition state.
6. Reaction Coordinate Diagram - VIZISCIENCE ... Reaction Coordinate Diagram. Given the following reaction, sketch a reaction coordinate graph. The reaction involves two steps, step 1 is the slowest step and ...
Energy Diagrams, Transition States, and Reactive ... Energy diagrams are used to represent the change in energy for the molecules involved in a chemical reaction. The free energy is measured along the y-axis, and the reaction coordinate is plotted on the x-axis.. The reaction coordinate indicates the progress of the conversion of reactants to products.


























0 Response to "45 labeled reaction coordinate diagram"
Post a Comment