43 two step reaction diagram
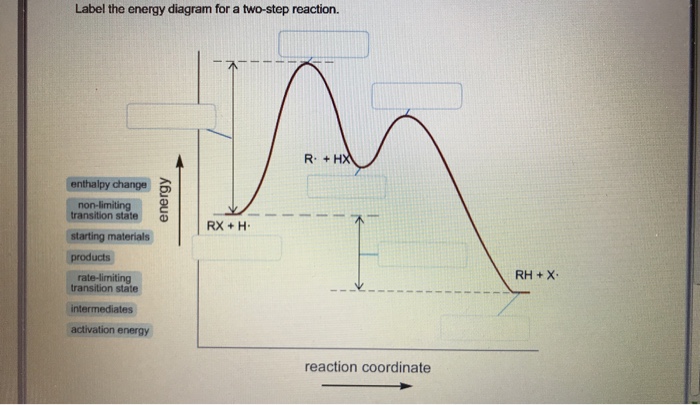
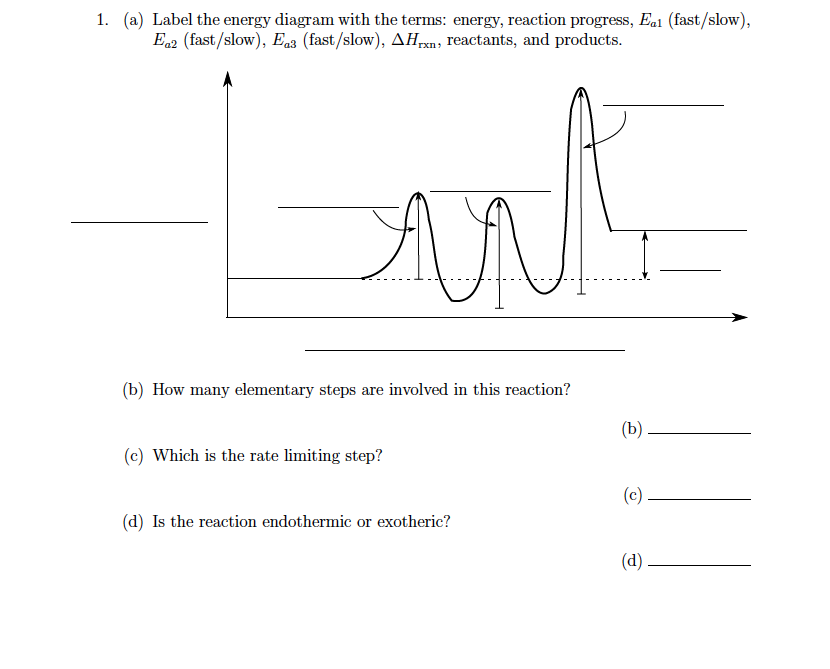
Energy Diagram: The diagram which indicates the number of steps in which the reaction gets completed and the energy required for each step can be shown by plotting graph of rate of reaction versus ... Recall that an energy diagram is usually read from left to right. The components of a two-step energy diagram are: • Reactants: are placed on the left/beginning of the energy diagram. • Products: are placed on the right/end of the energy diagram. • Non-limiting transition state: is the transition state with the lowest energy in the energy ...
The relative reaction rates and product branching ratios responded sensitively to the electronic property of the R group, providing key evidence to deriving a two-step reaction mechanism. The first step involved • OH conducting a back-side attack on one of the sulfur atoms, forming sulfenic acid (-SOH) and thiyl radical (-S •) product ...
Two step reaction diagram
Feb 03, 2020 · The activation energy for each step is labeled e a1 and e a2. Label the energy diagram for a two step reaction. It also shows the effect of a catalyst on the forward and reverse activation energy. In the s n 1 reaction the carbocation species is a reaction intermediate. A potential energy diagram for an s n 1 reaction shows that the carbocation ... Draw a reaction energy diagram for a two-step reaction that has an endothermic first step and an exothermic second step. Label the reactants, transition states, reaction intermediate, activation energies, and enthalpy differences. Step-by-step solution. 100% (4 ratings) for this solution. A two-step reaction mechanism is proposed for a gas-phase reaction, as represented above. Which of the following correctly identifies both the chemical equation for step 1 and the rate law for the overall reaction? ... The diagram below shows two reaction profiles (path one and path two) for the decomposition of XY2. ...
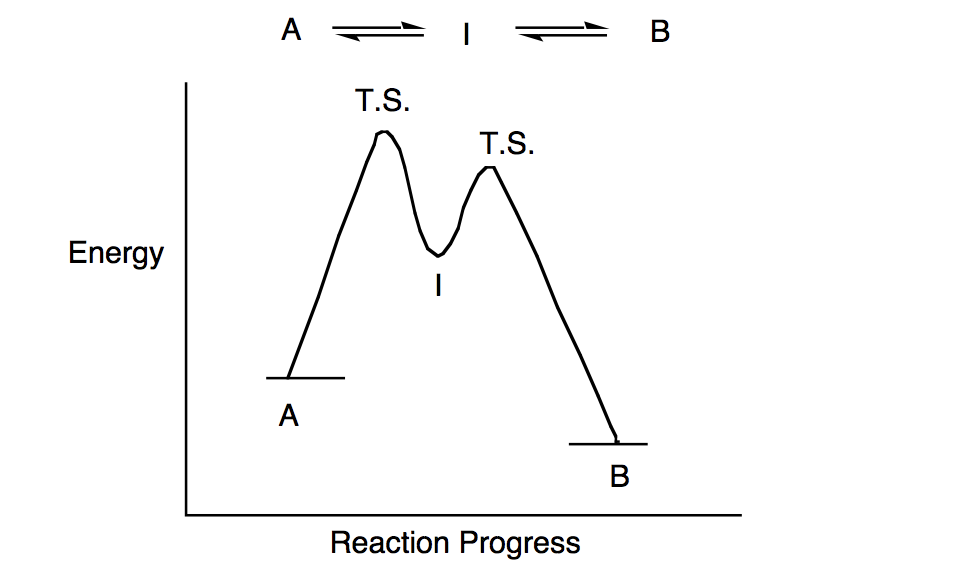
Two step reaction diagram. Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DG rxn, as well as DG 1 * and DG 2 * for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk. The diagram below shows the reaction progress of the two-step mechanism proposed. Notice how the reaction intermediate and the transition states do not last very long, but the intermediate can be isolated due to its fully formed bonds. Diagram of a Two-Step Reaction Mechanism Example with a Fast Reversible First Step then a Slow Step Summary. A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs. A reaction that occurs in two or more elementary steps is called a multistep or complex reaction. A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step. The catalyzed reaction is the one with lesser activation energy, in this case represented by diagram b. Check Your Learning Reaction diagrams for a chemical process with and without a catalyst are shown below. Both reactions involve a two-step mechanism with a rate-determining first step.
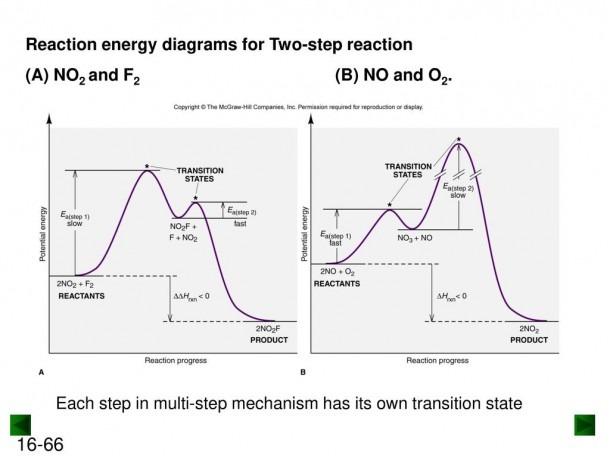
This two-step mechanism is illustrated for the reaction of ethene with hydrogen chloride by the following equations. First Step: H 2 C=CH 2 + HCl. H H 2 C=CH 2(+) + Cl (-) Second Step: H H 2 C=CH 2(+) + Cl (-) H H 2 C=CH 2 Cl. An energy diagram for this two-step addition mechanism is shown to the left. Figure 1. Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. The catalyzed pathway involves a two-step mechanism (note the presence of two transition states) and an intermediate species (represented by the valley between the two transitions states). • An energy diagram for a two-step reaction with one intermediate. Energy Diagrams. 9 • Organic chemists use a technique called electron pushing, alternatively called arrow pushing, to depict the flow of electrons during a chemical reaction. Label the energy diagram for a two-step reaction. Q. A reaction coordinate diagram is shown below for the reaction of A to form E. Answer the following questions.i) Identify the transition state (s)?ii) W... Q. Which reaction coordinate diagram represents a reaction in which the activation energy, Ea, is 50 kj.mol-1 and the ΔHrxn is -15 kj. mol-1?
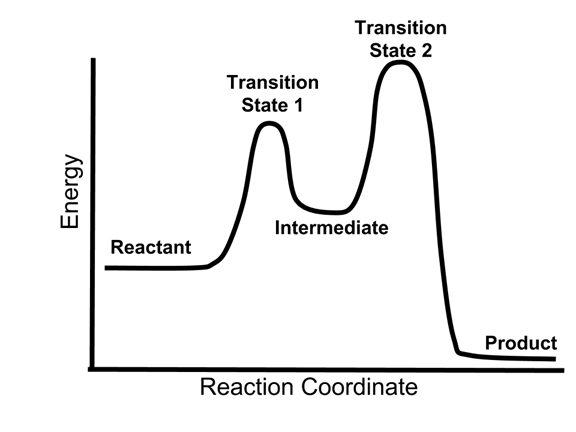
The E1 and S N 1 reactions always compete and a mixture of substitution and elimination products is obtained:. E1 - A Two-Step Mechanism. Let's break down the steps of the E1 reaction and characterize them on the energy diagram: Using Reaction Diagrams to Compare Catalyzed Reactions The two reaction diagrams here represent the same reaction: one without a catalyst and one with a catalyst. Identify which diagram suggests the presence of a catalyst, and determine the activation energy for the catalyzed reaction: Solution The energy diagram of a two-step reaction is shown below. In the above reaction, a reactant goes through one elementary step with a lower activation energy (transition state 1) to form the intermediate. The intermediate then goes through a second step (transition state 2) with the highest energy barrier to form the product. The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction.
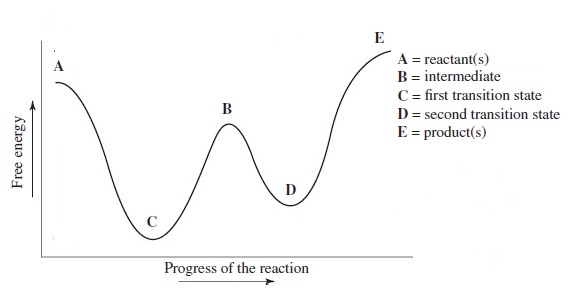
The reaction whose potential energy diagram is shown in the figure is a two-step reaction. The activation energy for each step is labeled E a1 and E a2 .Each elementary step has its own activated complex, labeled AC 1 and AC 2 .Note that the overall enthalpy change of the reaction is unaffected by the individual steps, since it depends only on the initial and final states.
Watch Complete videos @ www.LearningChemistryOnline.com Organic Chemistry 1
The rate determining step in a reaction mechanism is the slowest step. It is characterized by its high activation energy. Consider the energy diagram represented below of a two-step mechanism. The first step is the slow step since it has the highest activation energy. Here is more about this topic in the following video:
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Step 1: C2H4(g) + HCl(g) C2H5+(g) + Cl (g) rate-determining step Step 2: C2H5 +(g) + Cl (g) C 2H5Cl(g) fast step (c) Write the rate law for the reaction that is consistent with the reaction mechanism above. rate = k[C2H4][HCl] 1 point is earned for the correct rate law. (d) Identify an intermediate in the reaction mechanism above. C2H5 +(g) or ...
Oct 06, 2018 · Label the energy diagram for a two step reaction. Endothermic because energy is needed to break the a b bond. Each elementary step has its own activated complex labeled ac 1 and ac 2. It also shows the effect of a catalyst on the forward and reverse activation energy. Home study science chemistry chemistry questions and answers label the energy ...
The catalyzed reaction is the one with lesser activation energy, in this case represented by diagram (b). Check Your Learning. Reaction diagrams for a chemical process with and without a catalyst are shown below. Both reactions involve a two-step mechanism with a rate-determining first step.
Nov 19, 2021 · Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DG rxn, as well as DG 1 * and DG 2 * for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
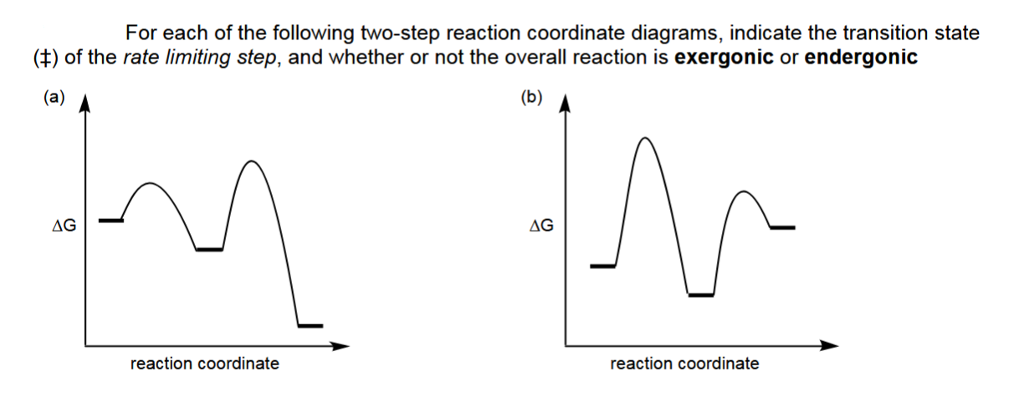
Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the second step has a higher-energy transition state than the first. Label the parts of the diagram corresponding to reactant, product, intermediate, overall ΔG ‡, and overall ΔG°.
As simple illustrations of stepwise mechanisms, we will consider two distinct scenarios for reactions which occur in two steps. First Step Rate-Determining. Mechanism: Reaction Path Diagram: The term "rate-determining step" or "rds" has a specific meaning. An rds is a step of a reaction the rate of which is equal to the rate of the ...

Unesco World Heritage Site, National Trust, Sunrise, Giant's Causeway, County Antrim, Northern Ireland.
The reaction involves two steps, step 1 is the slowest step and step 2 is the fastest step. Both steps are exothermic. Indicate on the diagram the overall enthalpy change of the reaction, the reaction for the transition states and intermediate states. The reaction is a reaction between hydrogen gas and brown vapor of iodine monochloride.
Energy Diagram for a Two-Step Reaction Mechanism Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and
A two-step reaction mechanism is proposed for a gas-phase reaction, as represented above. Which of the following correctly identifies both the chemical equation for step 1 and the rate law for the overall reaction? ... The diagram below shows two reaction profiles (path one and path two) for the decomposition of XY2. ...
Draw a reaction energy diagram for a two-step reaction that has an endothermic first step and an exothermic second step. Label the reactants, transition states, reaction intermediate, activation energies, and enthalpy differences. Step-by-step solution. 100% (4 ratings) for this solution.
Feb 03, 2020 · The activation energy for each step is labeled e a1 and e a2. Label the energy diagram for a two step reaction. It also shows the effect of a catalyst on the forward and reverse activation energy. In the s n 1 reaction the carbocation species is a reaction intermediate. A potential energy diagram for an s n 1 reaction shows that the carbocation ...







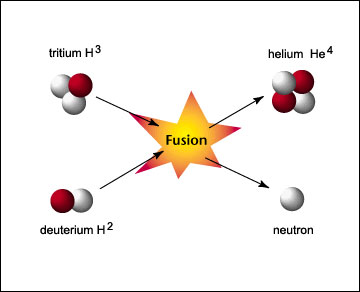











0 Response to "43 two step reaction diagram"
Post a Comment