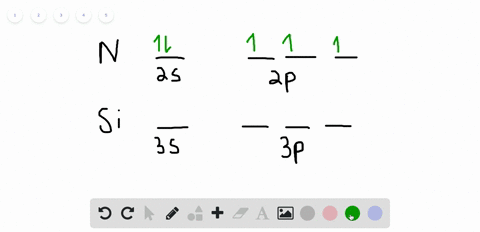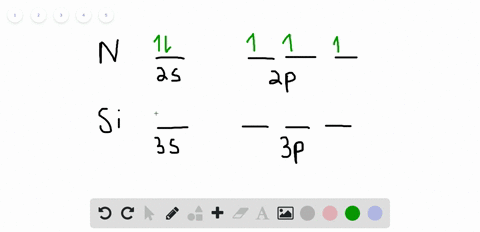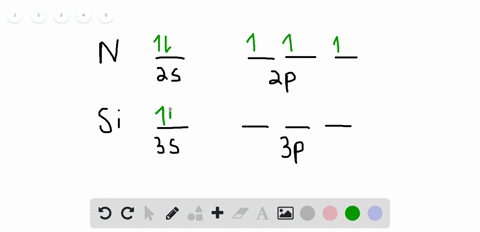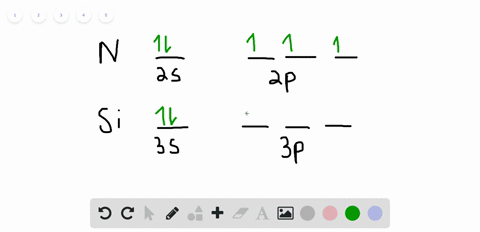
42 orbital filling diagram for silicon
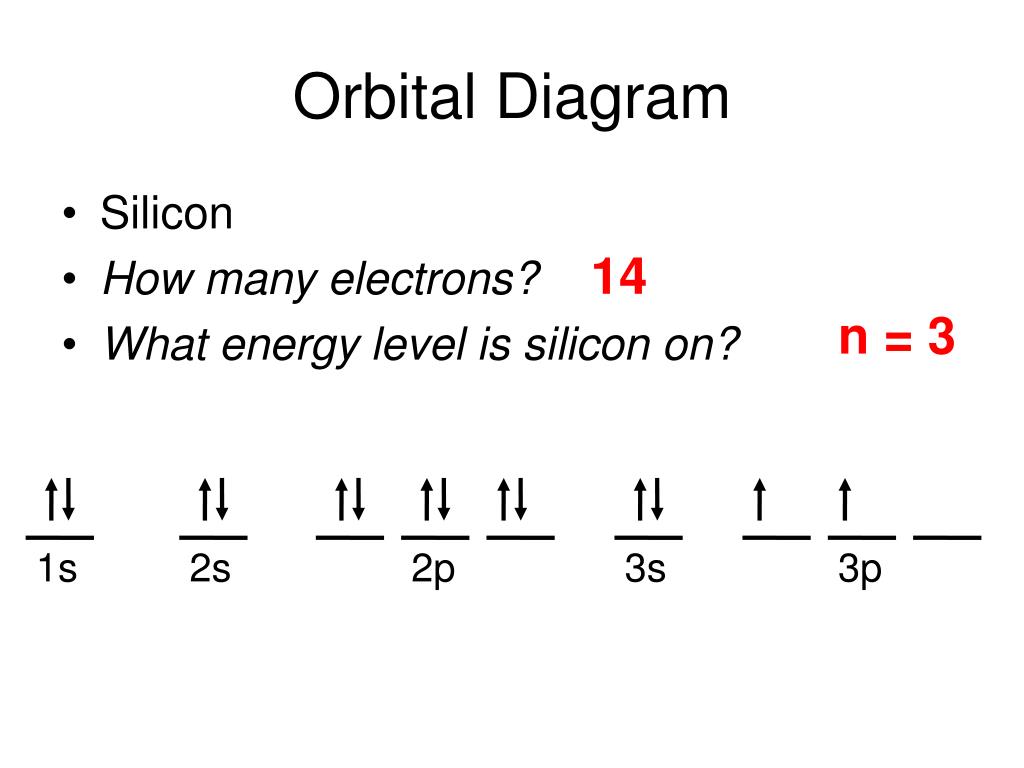
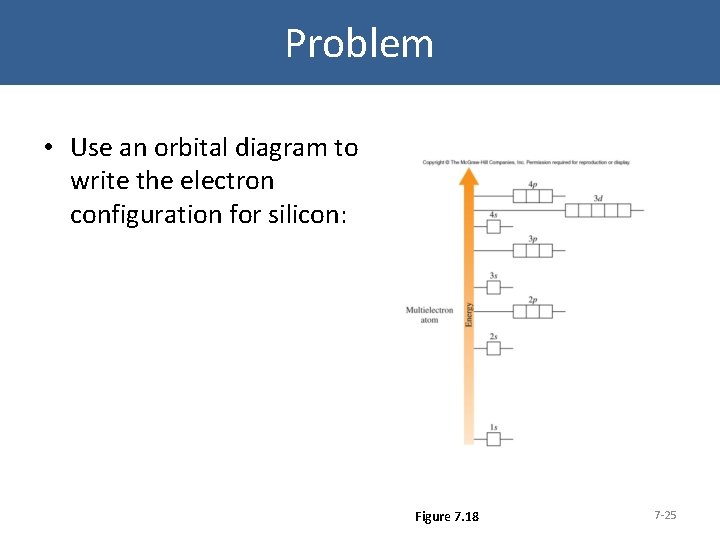
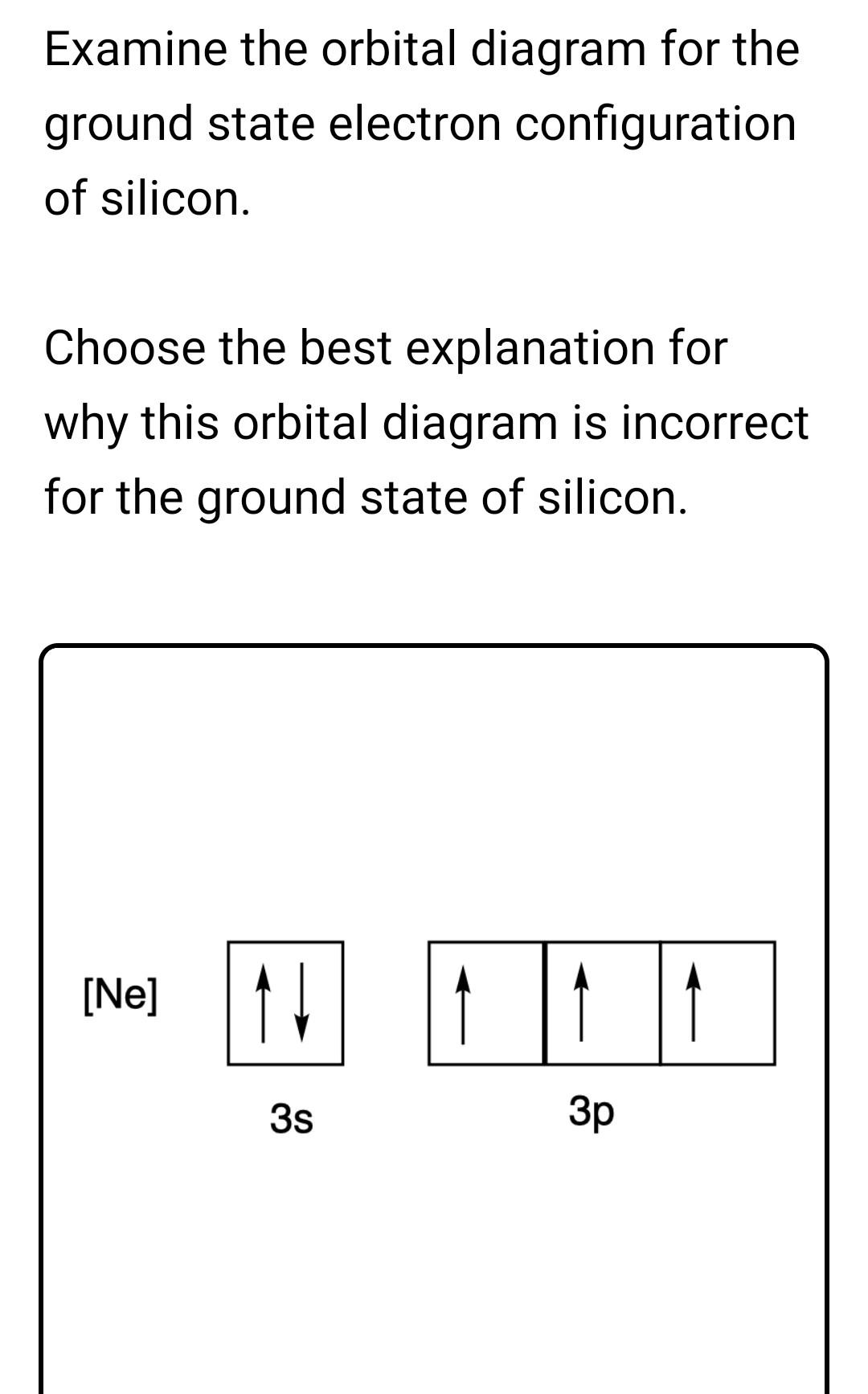
Electron Configuration for Silicon (Si) - UMD In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. Solved Fill in the orbital energy diagram for silicon Use ... Solved Fill in the orbital energy diagram for silicon Use | Chegg.com. Science. Chemistry. Chemistry questions and answers. Fill in the orbital energy diagram for silicon Use the elemento es importanti 3p A 35 E 2p 2s ls 1 item attempt remaining Try Another Version Submit Answer.
What is the orbital diagram for sulfur? - FindAnyAnswer.com Similarly one may ask, what is the orbital diagram for silicon? ... Bromine has 35 electrons, so it will have 35 arrows placed in its orbital-filling diagram as in figure 13. The order bottom to top, adding the electrons to the various sublevels, is 1s, 2s, 2p, 3s, 3p, 4s, 3d and 4p. Notice that the 3d sublevel is filled after the 4s and before ...
Orbital filling diagram for silicon
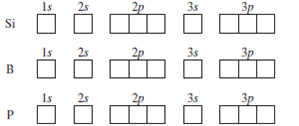
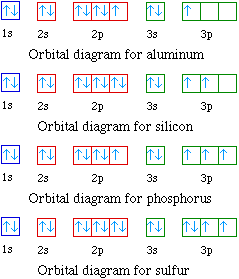
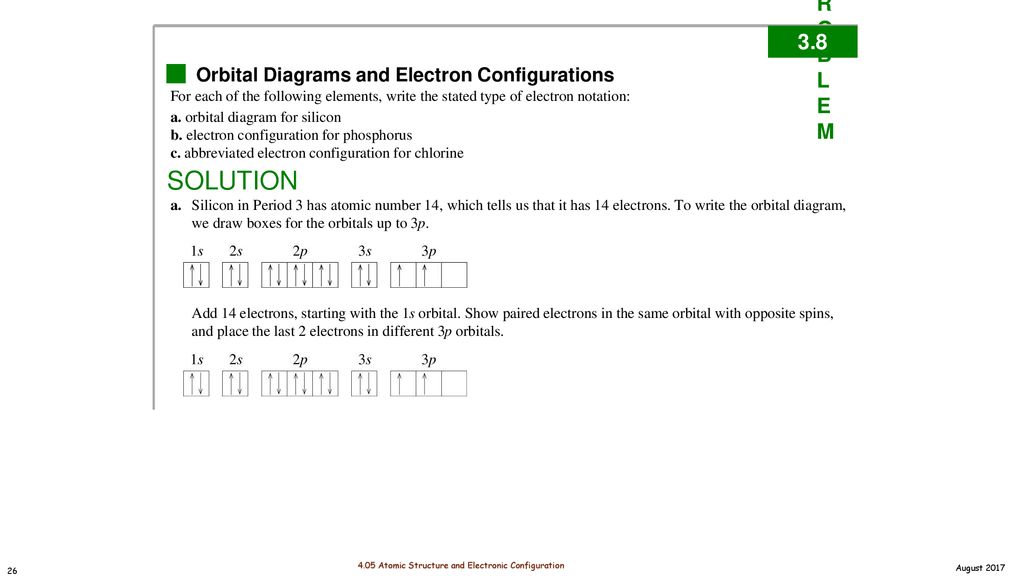
Electron configuration of silicon (Si), orbital diagram ... Orbital Diagram, electron configuration, and the noble gas notation for a silicon (Si) atom. PDF Electron Configuration and Orbital Filling Diagram WS Key Orbital filling diagram Silicon - Long e- config: 2 Short e- config: Orbital filling diagram 2s2z 3 G Gold - Long e- config: Short e- config: Xe - Long e- config: Short e- config: U short e-config: Oxygen long e-config: Ba short e-config: Pb long e-config: Label the adjacent pictures as highest & lowest frequency longest & shortest wavelength PDF Work on Elctron configuration and orbital diagram 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium
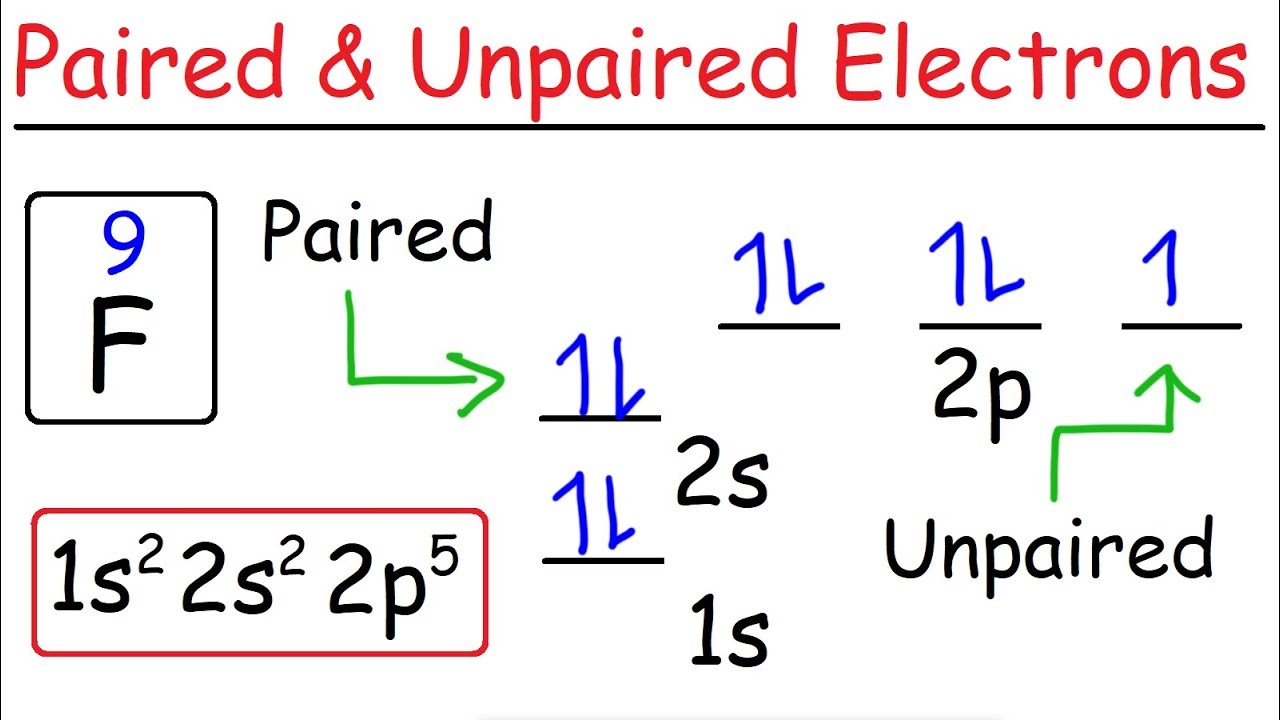
Orbital filling diagram for silicon. DOC Electron Configuration Practice Worksheet - Weebly 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams . for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Lewis Dot a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine g. Chromium h. Phosphorus Bromine PAGE THREE INDEPENDENT PRACTICE What do the arrows in orbital filling diagrams indicate? In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Each sublevel is labeled by its principal energy level and sublevel. Electrons are indicated by arrows inside the circles. Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15 ... Filling of Electrons in Orbitals: Definition, Properties ... The order of electron filling in the orbitals of the \(\text {n} = 1\) and \(\text {n} = 2\) shells is depicted in the diagram below. The orbitals are shown by circles, and the numbers typed in them reflect the sequence of filling for the first ten electrons.
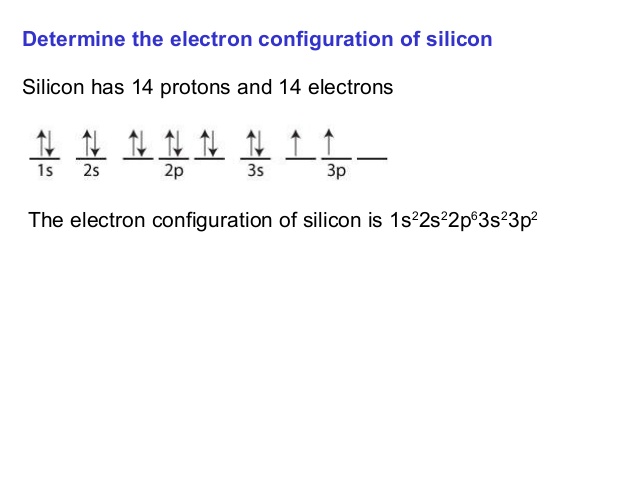
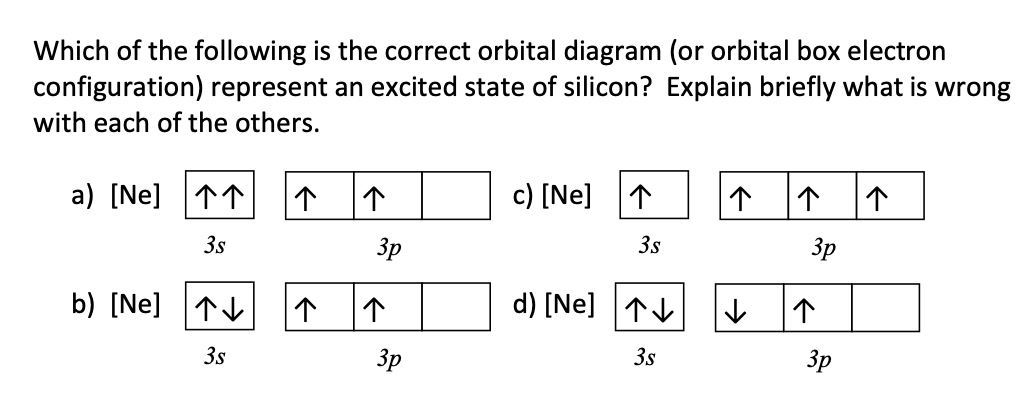
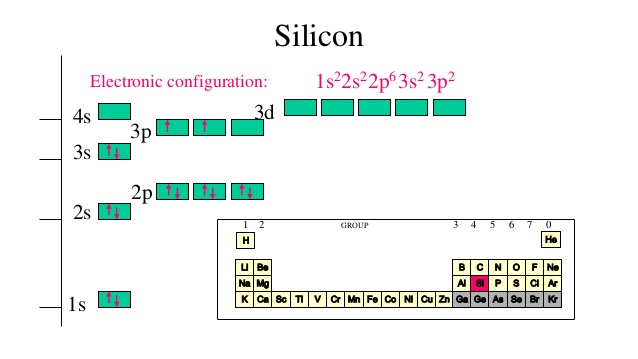
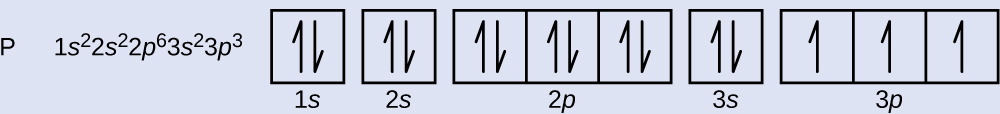
PDF Hund©s Rule & Orbital Filling Diagram - Math Worksheets 4 ... Hund©s Rule & Orbital Filling Diagram Complete the orbital diagram for each element. 2) calcium 1s 2s 4s 3s 3d 2p 4p 3p 1) sodium 1s 2s 4s 3s 3d 2p 4p 3p 3) nickel 1s 2s 4s 3s 3d 2p 4p 3p 4) silicon 1s 2s 4s 3s 3d 2p 4p 3p 5) iron 6) copper 1s 2s 4s 3s 3d 2p 4p Answer key 3p 2s2 2p6 3s2 3p6 4s2 1s 2s 4s 3s 2p 4p 3p 3d. Aluminum(Al) electron configuration with orbital diagram These orbitals are named s, p, d, f. The electron holding capacity of these orbitals is s = 2, p = 6, d = 10 and f = 14. The Aufbau electron configuration method is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d. The first two electrons of aluminum enter the 1s orbital. Silicon(Si) electron configuration and orbital diagram Orbital diagram for silicon (Si) Silicon (Si) excited state electron configuration Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of silicon is 1s2 2s2 2p6 3s2 3p2. The valency of the element is determined by electron configuration in the excited state. Orbital Filling Diagram For Sulfur Show the orbital-filling diagram for (bromine).Status: Resolved. Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top%(15). 1. Describe the two differences between a 2p x orbital and a 3p y orbital.
Orbital Diagram For Arsenic - schematron.org Orbital Diagram For Arsenic. Because the 4p section has 3 orbitals, but Arsenic ends with 4p3. It'll want to leave as few orbitals empty, so you have three arrows pointing up. The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org! PDF Electron Configuration Practice Worksheet - Weebly 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium Periodic Trends Flashcards - Quizlet Show the orbital-filling diagram for N (nitrogen). ... Give the ground-state electron configuration for silicon (Si) using noble-gas shorthand. [Ne]3s^23p^2. Item 3: Part C Give the actual ground-state electron configuration for copper (Cu) using the complete form. 1s^22s^22p^63s^23p^63d^104s^1. Silicon Orbital Diagram - ViralListClub.com Orbital diagrams must follow 3 rules. The orbitals 1s 2s 2p and 3s are filled first with 2 2 6 and 2 electrons respectively. The Orbital Diagram for Silicon. In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. The nex six electrons will go in the 2p orbital.
Solved Fill in the orbital energy diagram for silicon ... Answer to Solved Fill in the orbital energy diagram for silicon.
Hund's Rule and Orbital Filling Diagrams | Chemistry for ... An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally.
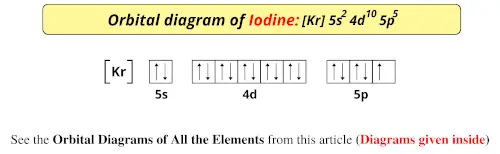
Orbital Filling Diagram For Bromine Orbital-Filling Diagram for Bromine. Bromine has 35 electrons, so it will have 35 arrows placed in its orbital-filling diagram as in figure The order bottom to top .Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top.
What is the orbital diagram for silicon? - Studyrankersonline In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital.
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
What is the orbital notation for carbon? Additionally, what is the orbital notation for iron? Thus, following the rules on how to fill the orbitals, the electronic configuration of iron (for example) is 1s2 2s2 2p6 3s2 3p6 4s2 3d6 , and it is abbreviated form [Ar] 4s2 3d6.However, I has not been easy to find the explanation on why in any periodic table it is written as [Ar] 3d6 4s2 instead.
Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
Electron Configuration & Orbital Filling Diagram Ws Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium Where are the Electrons? Write the full electron configuration, short-hand electron configuration, and fill in the orbital . Place the ending configuration and the dot diagram for the above ...
PDF Work on Elctron configuration and orbital diagram 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium
PDF Electron Configuration and Orbital Filling Diagram WS Key Orbital filling diagram Silicon - Long e- config: 2 Short e- config: Orbital filling diagram 2s2z 3 G Gold - Long e- config: Short e- config: Xe - Long e- config: Short e- config: U short e-config: Oxygen long e-config: Ba short e-config: Pb long e-config: Label the adjacent pictures as highest & lowest frequency longest & shortest wavelength
Electron configuration of silicon (Si), orbital diagram ... Orbital Diagram, electron configuration, and the noble gas notation for a silicon (Si) atom.



/aufbauexample-56a129555f9b58b7d0bc9f48.jpg)















0 Response to "42 orbital filling diagram for silicon"
Post a Comment